L9787
L-655,708
≥98% (HPLC), powder
동의어(들):
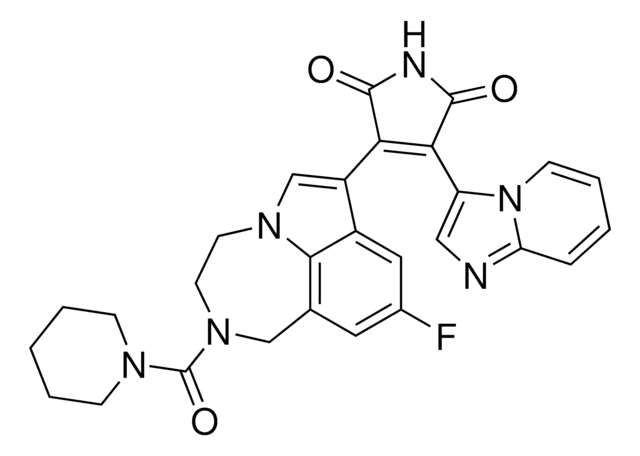
Ethyl (S)-11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate, L-655708
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C18H19N3O4
CAS Number:
Molecular Weight:
341.36
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
분석
≥98% (HPLC)
양식
powder
solubility
DMSO: ≥6.0 mg/mL (Warmed)
H2O: insoluble
주관자
Merck & Co., Inc., Kenilworth, NJ, U.S.
SMILES string
CCOC(=O)c1ncn2-c3ccc(OC)cc3C(=O)N4CCCC4c12
InChI
1S/C18H19N3O4/c1-3-25-18(23)15-16-14-5-4-8-20(14)17(22)12-9-11(24-2)6-7-13(12)21(16)10-19-15/h6-7,9-10,14H,3-5,8H2,1-2H3/t14-/m0/s1
InChI key
YKYOQIXTECBVBB-AWEZNQCLSA-N
유전자 정보
human ... GABRA5(2558)
관련 카테고리
애플리케이션
L-655,708 has been used as an α5 GABAA receptor inverse agonist to inhibit the discriminative stimulus of propofol in a dose-dependent manner.
생화학적/생리학적 작용
L-655,708 is an inverse agonist of the α5 γ-Aminobutyric acid type A (GABAA) receptor. It has an ability to increase cognition in rats.
Ligand for benzodiazepine site of GABAA receptors containing α5 subunits.
Novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit.
특징 및 장점
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the GABAA Receptors page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
This compound was developed by Merck & Co., Inc., Kenilworth, NJ, U.S.. To browse the list of other pharma-developed compounds and Approved Drugs/Drug Candidates, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
Flavia R Carreno et al.
The international journal of neuropsychopharmacology, 20(6), 504-509 (2017-03-25)
Selective augmentation of hippocampal activity in ways similar to that caused by ketamine may have therapeutic advantages over ketamine, which has psychotomimetic and reinforcing effects likely due to effects outside the hippocampus (i.e., off-target effects). Here we evaluated the antidepressant-like
C Sur et al.
Molecular pharmacology, 54(5), 928-933 (1998-11-06)
The gamma-aminobutyric acid (GABA)A receptor is a hetero-oligomer consisting of five subunits, the combination of which confers unique pharmacological properties to the receptor. To understand the physiological role of native GABAA receptors, it is critical to determine their subunit compositions.
L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors
Atack JR, et al.
Neuropharmacology, 51(6), 1023-1029 (2006)
M Xue et al.
European journal of pain (London, England), 21(6), 1061-1071 (2017-02-02)
γ-Aminobutyric acid (GABA) type A receptors (GABA The C fibre-evoked field potentials were recorded in superficial dorsal horn of spinal cord, and the effects of α5-GABA Inhibition of α5-GABA α5-GABA Tonic inhibition generated by α5-GABA
Jonathan Fischell et al.
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 40(11), 2499-2509 (2015-04-23)
Selective serotonin reuptake inhibitors (SSRIs) are the primary pharmacological treatment for depression, but SSRIs are effective in only half of the patients and typically take several weeks to relieve symptoms. The NMDA receptor antagonist ketamine exerts a rapid antidepressant action
관련 콘텐츠
DISCOVER Bioactive Small Molecules for Neuroscience
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.