추천 제품
재조합
expressed in baculovirus infected Sf9 cells
Quality Level
양식
buffered aqueous solution
분자량
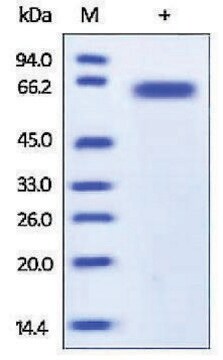
57 kDa
농도
≥2,000 unit/mL
UniProt 수납 번호
배송 상태
dry ice
저장 온도
−70°C
유전자 정보
human ... FURIN(5045)
애플리케이션
Furin is capable of cleaving precursors of a wide variety of proteins, including growth factors, serum proteins, including proteases of the blood-clotting and complement systems, matrix metalloproteinases, receptors, viral-envelope glycoproteins, and bacterial exotoxins, typically at sites marked by the consensus sequence Arg-Xaa-(Lys/Arg)-Arg.
생화학적/생리학적 작용
Furin is a dibasic endoprotease that is localized in the Golgi apparatus. It is responsible for the proteolytic maturation of many precursor proteins in the secretory and endocytic pathways of mammalian cells.
Furin is a dibasic endoprotease that is localized in the Golgi apparatus. It has a molecular mass of 52.7 kDa. It is responsible for the proteolytic maturation of many precursor proteins in the secretory and endocytic pathways of mammalian cells. Furin cleaves precursor proteins at their paired basic amino acid processing sites. Some substrates of furin include von Willebrand factor, transforming growth factor beta 1 precursor, pro-beta-secretase and proparathyroid hormone.
단위 정의
One unit is defined as the amount of enzyme required to cleave 25 μg of a MBP-FN-paramyosin-ΔSal substrate to 95% completion in 6 hours at 25°C in a total reaction volume of 25 μl.
물리적 형태
Solution in 10 mM MES, pH 7.0 at 25 °C, 1 mM CaCl2, 50% glycerol.
제조 메모
Isolated from Spodoptera frugiperda (Sf9) cells infected with recombinant baculovirus carrying truncated human furin
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
D A Bravo et al.
The Journal of biological chemistry, 269(41), 25830-25837 (1994-10-14)
Maturation of the insulin proreceptor in a late Golgi compartment requires cleavage at an Arg-Lys-Arg-Arg processing site, suggesting involvement of furin, a transmembrane serine protease of the Kex2 family of processing enzymes. A genetically engineered secreted, soluble form of human
Katherine L Hussmann et al.
The Journal of general virology, 95(Pt 9), 1991-2003 (2014-06-13)
The molecular basis for the increased resistance of astrocytes to a non-neuropathogenic strain of West Nile virus (WNV), WNV-MAD78, compared with the neuropathogenic strain WNV-NY remains unclear. Here, we demonstrated that the reduced susceptibility of astrocytes to WNV-MAD78 is due
Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site
R.J. Wise et al.
Proceedings of the National Academy of Sciences of the USA, 87, 9378-9382 (1991)
Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15
M.C. Kiefer et al.
Dna and Cell Biology, 10, 757-769 (1992)
ANGPTL4 sensitizes lipoprotein lipase to PCSK3 cleavage by catalyzing its unfolding.
Anne-Marie Lund Winther et al.
Journal of lipid research, 62, 100071-100071 (2021-03-28)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.