모든 사진(1)
About This Item
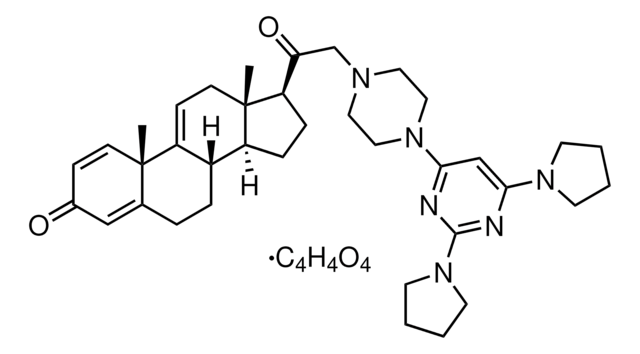
실험식(Hill 표기법):
C20H24O2
CAS Number:
Molecular Weight:
296.40
Beilstein:
1798411
MDL number:
UNSPSC 코드:
12352106
PubChem Substance ID:
NACRES:
NA.77
추천 제품
생물학적 소스
synthetic (organic)
분석
≥97%
양식
powder
mp
78-80 °C
solubility
ethanol: 50 mg/mL, clear, colorless to yellow
저장 온도
−20°C
SMILES string
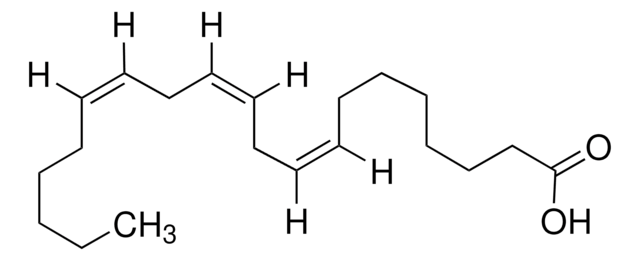
CCCCCC#CCC#CCC#CCC#CCCCC(O)=O
InChI
1S/C20H24O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h2-5,8,11,14,17-19H2,1H3,(H,21,22)
InChI key
MGLDCXPLYOWQRP-UHFFFAOYSA-N
유전자 정보
human ... ALOX15(246)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
Eicosatetraynoic acid (ETYA) is a non-metabolizable analog of ω-6 arachidonic acid. ETYA is a strong activator of the human peroxisome proliferator-activated receptor α (PPARα). It acts as an inhibitor of lipoxygenases (LOX) and cyclooxygenases (COX).
Eicosatetraynoic acid is a lipoxygenase and cyclooxygenase inhibitor.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
A S Taylor et al.
Prostaglandins, 29(3), 449-458 (1985-03-01)
5,8,11,14-eicosatetraynoic acid (ETYA), a widely used inhibitor of cyclooxygenase and lipoxygenase, inhibited the incorporation of 14C-arachidonic acid into cell lipids of the murine thymoma EL4 whereas oleic acid had no effect. Inhibition appeared to result from the ability of ETYA
Günther F E Scherer et al.
FEBS letters, 581(22), 4205-4211 (2007-08-19)
Auxin increases phospholipase A(2) activity within 2min (Paul, R., Holk, A. and Scherer, G.F.E. (1998) Fatty acids and lysophospholipids as potential second messengers in auxin action. Rapid activation of phospholipase A(2) activity by auxin in suspension-cultured parsley and soybean cells.
Parissa Taheri et al.
Journal of plant physiology, 167(3), 201-208 (2009-09-05)
Vitamins are plant growth regulators and activators of defense responses against pathogens. The cytomolecular mechanisms involved in the induction of resistance by chemicals especially vitamins on monocotyledonous plants are largely unknown. Here, we show that riboflavin, which acts as a
Miriam Guizy et al.
American journal of physiology. Cell physiology, 289(5), C1251-C1260 (2005-07-01)
Dietary polyunsaturated fatty acids (PUFAs) have been reported to exhibit antiarrhythmic properties, which have been attributed to their availability to modulate Na(+), Ca(2+), and several K(+) channels. However, their effects on human ether-a-go-go-related gene (HERG) channels are unknown. In this
Kazuhiro Tamura et al.
Vascular pharmacology, 44(6), 411-416 (2006-05-03)
To address the role of prostaglandin E2 (PGE2) in tube formation of endothelial cells and the relationships between the action of PGE2 and vascular endothelial growth factor (VEGF), cultured human umbilical vein endothelial cells (HUVECs) were used to evaluate tube
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.