E1636
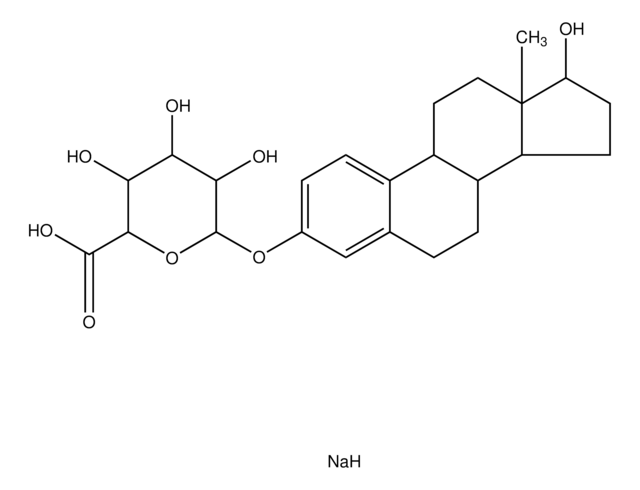
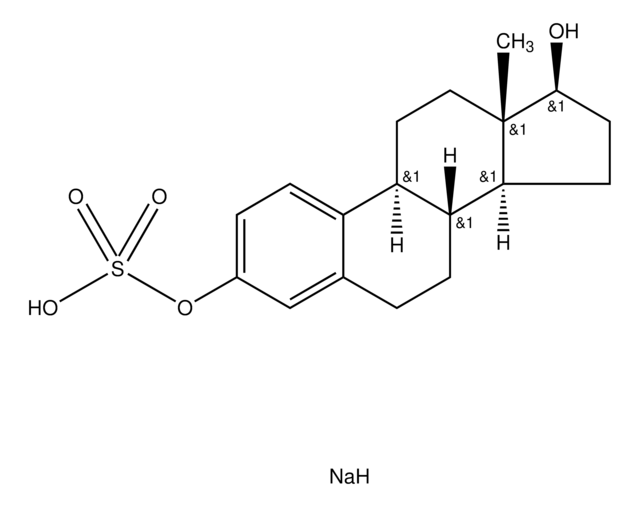
β-Estradiol 3,17-disulfate dipotassium salt
≥95%
동의어(들):
1,3,5(10)-Estratriene 3,17β-disulfate, 3,17β-Dihydroxy-1,3,5(10)-estratriene disulfate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C18H22O8S2K2
CAS Number:
Molecular Weight:
508.69
MDL number:
UNSPSC 코드:
51111800
PubChem Substance ID:
NACRES:
NA.77
생물학적 소스:
synthetic
양식:
powder
분석:
≥95%
추천 제품
생물학적 소스
synthetic
분석
≥95%
양식
powder
기술
inhibition assay: suitable
solubility
water: 50 mg/mL, clear, colorless
배송 상태
ambient
저장 온도
−20°C
SMILES string
[K].[H][C@]12CC[C@]3(C)[C@H](CC[C@@]3([H])[C@]1([H])CCc4cc(OS(O)(=O)=O)ccc24)OS(O)(=O)=O
InChI
1S/C18H24O8S2.K.H/c1-18-9-8-14-13-5-3-12(25-27(19,20)21)10-11(13)2-4-15(14)16(18)6-7-17(18)26-28(22,23)24;;/h3,5,10,14-17H,2,4,6-9H2,1H3,(H,19,20,21)(H,22,23,24);;/t14-,15-,16+,17+,18+;;/m1../s1
InChI key
CPIZVUIARARPNO-LBARCDFESA-N
생화학적/생리학적 작용
Among other effects, this conjugated estrogen selectively inhibits glutathione S-transferases.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
J J Barycki et al.
Archives of biochemistry and biophysics, 345(1), 16-31 (1997-09-01)
3beta-(Iodoacetoxy)dehydroisoandrosterone (3beta-IDA), an analogue of the electrophilic substrate, Delta5-androstene-3,17-dione, as well as an analogue of several other steroid inhibitors of glutathione S-transferase, was tested as an affinity label of rat liver glutathione S-transferase, isozyme 1-1. A time-dependent loss of enzyme
Angelo Quaranta et al.
PloS one, 4(11), e7699-e7699 (2009-11-06)
Dramatic declines in amphibian populations have been described all over the world since the 1980s. The evidence that the sensitivity to environmental threats is greater in amphibians than in mammals has been generally linked to the observation that amphibians are
J D Bain et al.
Steroids, 43(6), 603-619 (1984-06-01)
The efficiencies for estrogen conjugate hydrolysis were compared between enzyme hydrolysis, acid solvolysis and a new method, ammonolysis. Samples included: 1) crystalline 1,3,5(10)-estratriene-3, 17 beta-diol disulfate (estradiol 3,17-disulfate), 2) squirrel monkey urine collected following an intravenous injection of [2,4,6,7-H] 1,3,5(10)-estratriene-3,17
A H Maas et al.
Nederlands tijdschrift voor geneeskunde, 145(2), 65-69 (2001-02-28)
Coronary heart disease develops on average 10-15 years later in women than in men and is uncommon before menopause. 17 beta-estradiol has atheroprotective properties through rapid vasodilatory effects on the endothelium by stimulating nitric monoxide production and longer-term actions by
Fanfan Zhou et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 27(5), 518-523 (2005-11-01)
Human organic anion transporter 4 (hOAT4) belongs to a family of organic anion transporters which play critical roles in the body disposition of clinically important drugs, including anti-HIV therapeutics, anti-tumor drugs, antibiotics, anti-hypertensives, and anti-inflammatories. hOAT4 is expressed in the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.