추천 제품
생물학적 소스
human
Quality Level
재조합
expressed in baculovirus infected insect cells (BTI-TN-5B1-4)
형태
solution
분자량
58 kDa
포장
vial of 0.5 nmol
UniProt 수납 번호
응용 분야
cell analysis
배송 상태
dry ice
저장 온도
−70°C
유전자 정보
human ... CYP1A2(1544)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Human cytochrome P450 has been used in a study to investigate non-viral environmental risk factors for nasopharyngeal carcinoma. Human cytochrome P450 has also been used in a study to investigate the dynamics and hydration of the active sites of mammalian cytochromes.
생화학적/생리학적 작용
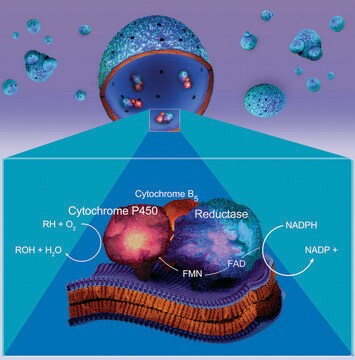
Cytochrome P450 enzymes are heme containing monooxygenases which are found primarily in the mammalian liver. They catalyze the oxidative metabolism of xenobiotics. This metabolism is the initial step in the biotransformation and elimination of a wide variety of drugs and environmental pollutants from the body. This product has a molecular mass of approximately 58 kDa. Amiodarone, furafylline, and cimetidine are inhibitors, whereas tobacco, insulin, and omeprazole are inducers of the enzyme. The isozyme CYP1A2 is a major pathway for the 6-hydroxylation of melatonin in vivo. This isozyme also catalyzes the 5-hydroxylation of thiabendazole.
Cytochrome P450 is a heterogeneous family of isozymes whose primary function is to oxidize small molecules, both as a function of intermediary metabolism (e.g., fatty acids) and to detoxify exogenous compounds (drugs or toxins). Some isoforms have narrow substrate specificity, while others are promiscuous. The CYP1A1 isoform catalyzes 7-deethylation of ethoxyresorufin. Cytochrome P450 (CYP) plays an important role in detoxifying xenobiotics, cellular metabolism and homeostasis. One of the main mechanisms of drug-drug interactions is the induction or inhibition of these enzymes. CYP enzymes are transcriptionally activated by a variety of xenobiotics and by endogenous substrates via receptor-dependent pathways. Inhibition of these enzymes is a major factor in metabolism-based drug-drug interactions, and many chemotherapeutic medications can cause drug interactions by either inhibiting or inducing the cytochrome p450 enzyme system.
기타 정보
Microsomes containing recombinant human CYP1A2, recombinant rabbit NADPH-P450 reductase, and cytochrome b5.
물리적 형태
Solution in 100 mM potassium phosphate buffer, pH 7.4.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Tereza Hendrychova et al.
Current drug metabolism, 13(2), 177-189 (2012-01-03)
The flexibility, active site volume, solvation, and access path dynamics of six metabolically active mammalian cytochromes P450 (human 2A6, 2C9, 2D6, 2E1, 3A4 and rabbit 2B4) are extensively studied using molecular dynamics (MD) simulations. On average, the enzymes' overall structures
Li Li et al.
Protein and peptide letters, 19(1), 57-61 (2011-09-17)
Human cytochrome P450(CYP 450) enzymes mediate over 60% of the phase I-dependent metabolism of clinical drugs. They are also known for the polymorphism functions that have significant impacts on the enzyme activities. In this study, a web-server called SCYPPred was
Wei-Hua Jia et al.
Seminars in cancer biology, 22(2), 117-126 (2012-02-09)
This review aims to systematically summarize the epidemiological studies on nasopharyngeal carcinoma (NPC) conducted over the past half century, covering descriptive epidemiological studies and reports on non-viral risk factors. Multiple lines of epidemiologic evidence for established risk factors are systematically
Moritz Walter et al.
Toxicology in vitro : an international journal published in association with BIBRA, 59, 215-220 (2019-04-21)
Next to its well-studied toxicity, carbon monoxide (CO) is recognized as a signalling molecule in various cellular processes. Thus, CO-releasing molecules (CORMs) are of considerable interest for basic research and drug development. Aim of the present study was to investigate
Xiangrong Zhang et al.
PloS one, 9(4), e94962-e94962 (2014-04-17)
The present study characterized in vitro metabolites of 20(R)-25-methoxyl-dammarane-3β, 12β, 20-triol (20(R)-25-OCH3-PPD) in mouse, rat, dog, monkey and human liver microsomes. 20(R)-25-OCH3-PPD was incubated with liver microsomes in the presence of NADPH. The reaction mixtures and the metabolites were identified
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.