추천 제품
생물학적 소스
mouse
Quality Level
결합
unconjugated
항체 형태
ascites fluid
항체 생산 유형
primary antibodies
클론
BAM-10, monoclonal
포함
15 mM sodium azide
종 반응성
human
향상된 검증
independent
Learn more about Antibody Enhanced Validation
기술
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:2,000 using formic acid-treated, formalin-fixed, human Alzheimer′s disease (AD) brain sections.
indirect ELISA: suitable
동형
IgG1
배송 상태
dry ice
저장 온도
−20°C
타겟 번역 후 변형
unmodified
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
β-amyloid protein or aβ4 is derived from larger protein that belongs to the family of 70kDa transmembrane glycoproteins (amyloid precursor proteins, APP). These are produced in various isoforms by alternative splicing. APPs are synthesized by many tissues including brain cells. Abnormal β-amyloid protein deposits have been associated with Alzheimer′s disease, Down′s syndrome, Dutch-type amyloidosis and Lewy body dementia.
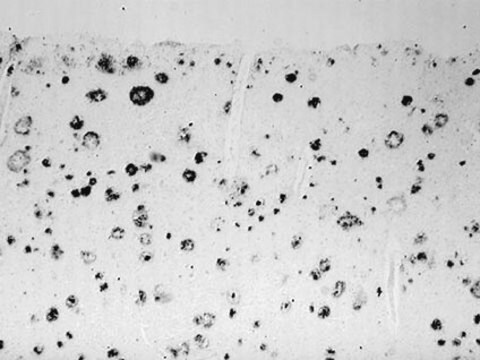
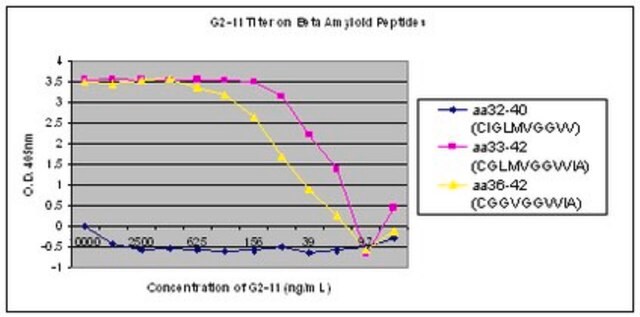
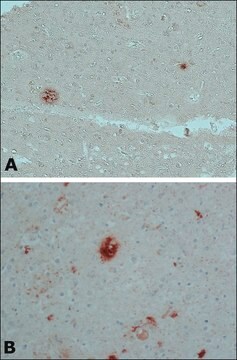
The antibody reacts specifically with β-amyloid protein. The epitope recognized by the antibody resides within amino acids 1-12 of the β-amyloid protein. It specifically stains amyloid plaques within the cortex and amyloid deposits in blood vessels using formic acid-treated, formalin-fixed, paraffin-embedded, and Methacarn-fixed sections of human Alzheimer′s disease (AD) brain tissue.
특이성
Monoclonal Anti-β-Amyloid Protein reacts specifically with β-amyloid protein. The epitope recognized by the antibody resides within amino acid residues 1-12 of the β-amyloid protein. The antibody specifically stains amyloid plaques within the cortex, and amyloid deposits in blood vessels, in formic acid-treated, formalin-fixed, paraffin-embedded and Methacarn-fixed sections of human Alzheimer′s disease (AD) brain tissue.
면역원
Synthetic β-amyloid peptide, conjugated to KLH.
애플리케이션
Monoclonal Anti- β Amyloid Protein may be used for the localization of β -amyloid protein using various immunochemical assays such as ELISA, competitive ELISA and immunohistochemistry.
Mouse monoclonal anti-ABETA was used to treat old WT PDAPP mice with amyloid accumulation and learning deficits in an attempt to improve learning and decrease accumulation, however no response was observed.
The antibody is useful in immunohistochemistry, immunoblotting, ELISA, and competitive ELISA. Also, this antibody has been used to neutralize Aβ assemblies in brains of transgenic mice expressing a mutant form of amyloid precursor protein, and for in vivo deep tissue imaging using near-IR optical spectrum.
생화학적/생리학적 작용
β-amyloid fragments are amyloidogenic and neurotoxic both in vitro and in vivo. The presence of a large number of neuritic (senile) plaques and neurofibrillary tangles in the cerebral cortex is used as a pathological marker for a disease state and presents the major criterion for the diagnosis of Alzheimer′s disease at autopsy. A monoclonal antibody reacting specifically with β-amyloid protein is valuable for studying the nature of the β-amyloid protein by enabling detection and localization of β-amyloid protein and fragments.
물리적 형태
Monoclonal Anti-β-Amyloid Protein is provided as ascites fluid with 15mM sodium azide as a preservative.
저장 및 안정성
For continuous use, store at 2-8 °C for no more than one month. For extended storage, freeze in working aliquots. Repeated freezing and thawing is not recommended. Storage in "frost-free" freezers is not recommended. If slight turbidity occurs upon prolonged storage, clarify the solution by centrifugation before use.
면책조항
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
관련 제품
제품 번호
설명
가격
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
Xian-Hui Dong et al.
Experimental and therapeutic medicine, 9(4), 1319-1327 (2015-03-18)
Alzheimer's disease (AD) is a neurodegenerative brain disorder and the most common cause of dementia. New treatments for AD are required due to its increasing prevalence in aging populations. The present study evaluated the effects of the active components of
Deficits in object-in-place but not relative recency performance in the APPswe/PS1dE9 mouse model of Alzheimer?s disease: Implications for object recognition
Bonardi, C, et al.
Behavioural Brain Research, 313, 71-81 (2016)
Effects of Folic Acid on Secretases Involved in Aβ Deposition in APP/PS1 Mice
Tian, T, et al.
Nutrients, 8(9), 556-556 (2016)
Use-dependent effects of amyloidogenic fragments of β-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo
Kim, J, et al.
The Journal of Neuroscience, 21(4), 1327-1333 (2001)
Tian Tian et al.
Nutrients, 8(9) (2016-09-13)
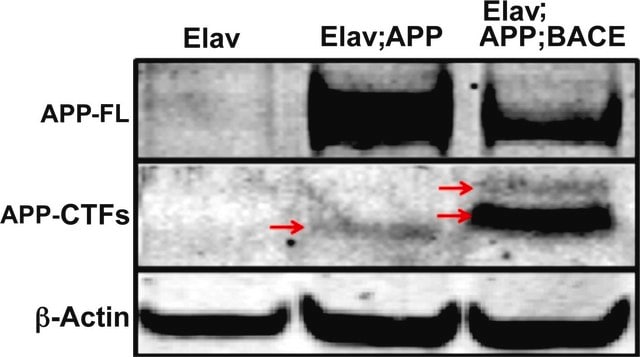
Alzheimer's disease (AD) is the most common type of dementia. Amyloid-β protein (Aβ) is identified as the core protein of neuritic plaques. Aβ is generated by the sequential cleavage of the amyloid precursor protein (APP) via the APP cleaving enzyme
문서
Alzheimer's disease (AD) is the most common cause of dementia in the elderly and is characterized by gradual loss of cognitive functions.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.