모든 사진(1)
About This Item
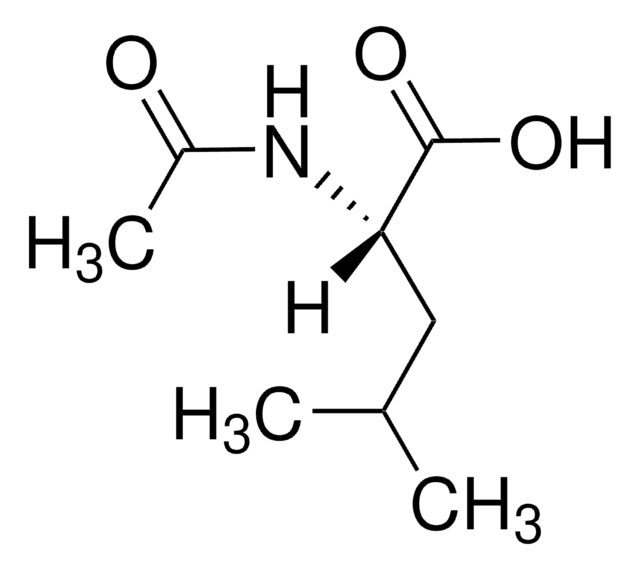
실험식(Hill 표기법):
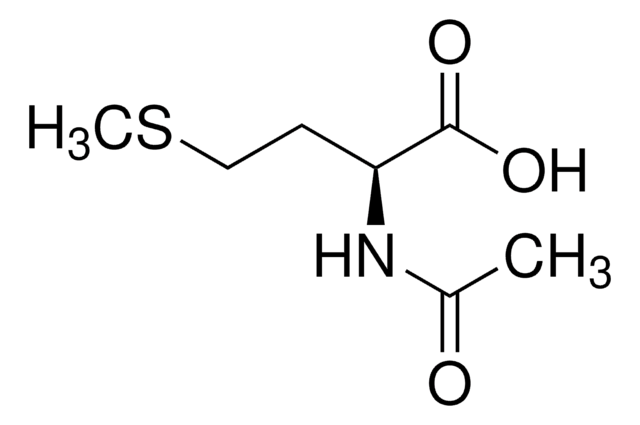
C7H13NO3S
CAS Number:
Molecular Weight:
191.25
Beilstein:
1725553
EC Number:
MDL number:
UNSPSC 코드:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
추천 제품
product name
N-Acetyl-D-methionine, ~99%
분석
~99%
형태
powder or crystals
기술
ligand binding assay: suitable
색상
white
mp
102.3-103.6 °C
저장 온도
−20°C
SMILES string
CSCC[C@@H](NC(C)=O)C(O)=O
InChI
1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m1/s1
InChI key
XUYPXLNMDZIRQH-ZCFIWIBFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
N-Acetyl-D-methionine may be used as a substrate to identify, differentiate and characterized N-acylamino acid racemase(s) and N-acyl-D-amino acid amidohydrolase(s).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Wen-Ching Wang et al.
Journal of molecular biology, 342(1), 155-169 (2004-08-18)
N-acylamino acid racemase (NAAAR) catalyzes the racemization of N-acylamino acids and can be used in concert with an aminoacylase to produce enantiopure alpha-amino acids, a process that has potential industrial applications. Here we have cloned and characterized an NAAAR homologue
Pei-Hsun Lin et al.
European journal of biochemistry, 269(19), 4868-4878 (2002-10-02)
An N-acyl-d-amino acid amidohydrolase (N-D-AAase) was identified in cell extracts of a strain, Iso1, isolated from an environment containing N-acetyl-d-methionine. The bacterium was classified as Variovorax paradoxus by phylogenetic analysis. The gene was cloned and sequenced. The gene consisted of
S Pittelkow et al.
Protein expression and purification, 12(2), 269-276 (1998-03-31)
Aminoacylase I (EC 3.5.1.14) is one of the most abundant enzymes in the cortical region of mammalian kidney. Both the porcine and the human enzyme were overexpressed using baculovirus expression vector systems and purified by hydrophobic interaction chromatography and anion-exchange
T Odajima et al.
Cell biochemistry and function, 16(2), 139-147 (1998-06-24)
Urate oxidase from Candida utilis, an enzyme containing an essential thiol, was examined for its sensitivity to lactoperoxidase, an oxidant present in breast milk. Upon exposure to a system composed of lactoperoxidase, hydrogen peroxide and bromide at moderately alkaline pH
M J Wick et al.
Biochemical pharmacology, 37(7), 1225-1231 (1988-04-01)
Both N-hydroxy-2-acetamidofluorene (N-OH-AAF) and the heterocyclic analogue, 2-(N-hydroxyacetamido)carbazole (N-OH-AAC), were shown to be mechanism-based irreversible inhibitors (suicide inhibitors) of partially purified rat hepatic N-acetyltransferase (NAT) activity. Although N-OH-AAC exhibited an apparent first-order inactivation rate constant (ki) that was 7-fold lower
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.