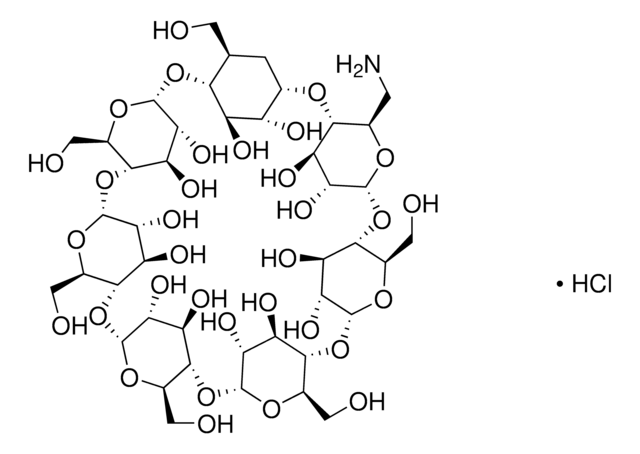
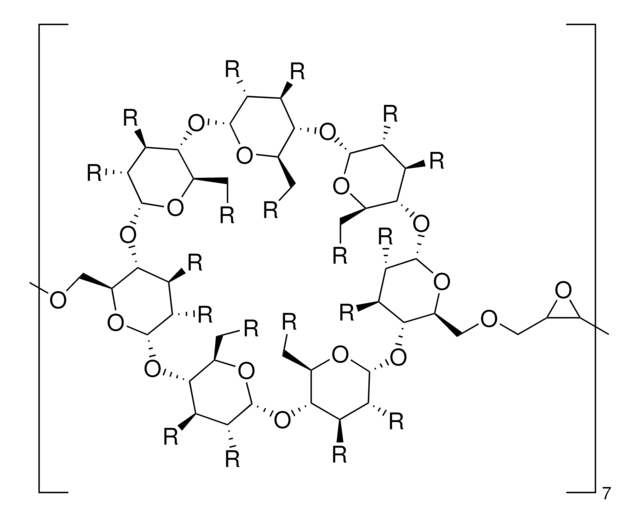
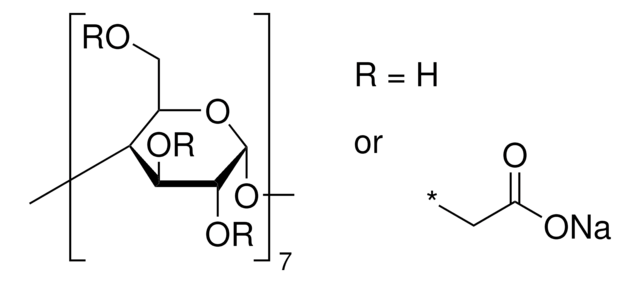
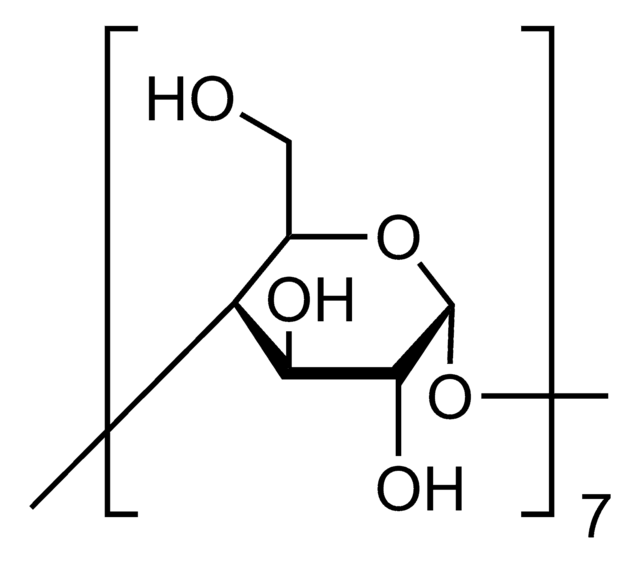
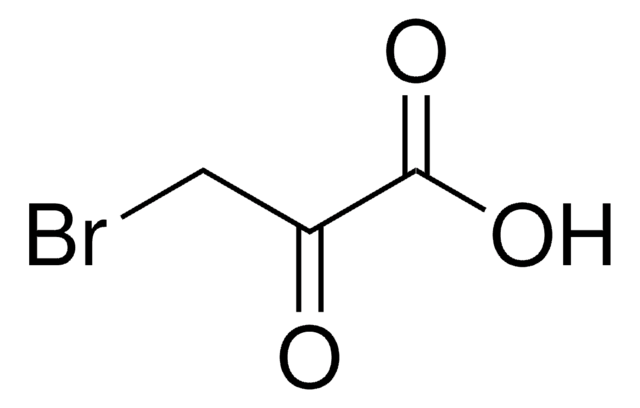
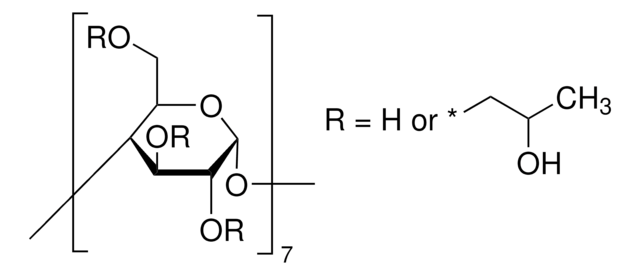
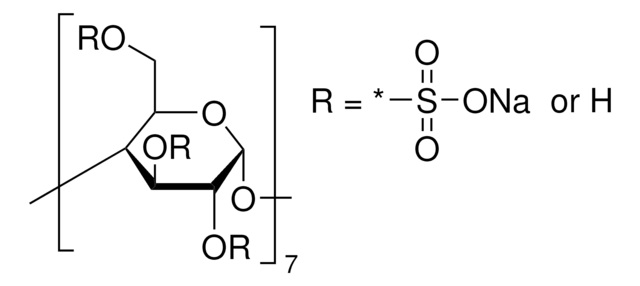
추천 제품
양식
powder
Quality Level
불순물
~5% water
색상
white
mp
225 °C ((437 °F ) - Decomposes on heating)
저장 온도
−20°C
SMILES string
CC(=O)CCC(=O)OC[C@H]1O[C@@H]2O[C@H]3[C@@H](O)[C@H](O)[C@H](O[C@@H]3COC(=O)CCC(O)=O)O[C@H]4[C@@H](O)[C@H](O)[C@H](O[C@@H]4COC(=O)CCC(O)=O)O[C@H]5[C@@H](O)[C@H](O)[C@H](O[C@@H]5COC(=O)CCC(O)=O)O[C@H]6[C@@H](O)[C@H](O)[C@H](O[C@@H]6COC(=O)CCC(O)=O)O[C@H]7[C@@H](O)[C@H](O)[C@H](O[C@@H]7COC(=O)CCC(O)=O)O[C@H]8[C@@H](O)[C@H](O)[C@H](O[C@@H]8COC(=O)CCC(O)=O)O[C@H]1[C@@H](O)[C@@H]2O
InChI
1S/C71H100O55/c1-23(72)2-9-37(85)106-16-24-58-44(92)51(99)65(113-24)121-59-25(17-107-38(86)10-3-31(73)74)115-67(53(101)46(59)94)123-61-27(19-109-40(88)12-5-33(77)78)117-69(55(103)48(61)96)125-63-29(21-111-42(90)14-7-35(81)82)119-71(57(105)50(63)98)126-64-30(22-112-43(91)15-8-36(83)84)118-70(56(104)49(64)97)124-62-28(20-110-41(89)13-6-34(79)80)116-68(54(102)47(62)95)122-60-26(18-108-39(87)11-4-32(75)76)114-66(120-58)52(100)45(60)93/h24-30,44-71,92-105H,2-22H2,1H3,(H,73,74)(H,75,76)(H,77,78)(H,79,80)(H,81,82)(H,83,84)/t24-,25-,26-,27-,28-,29-,30-,44+,45+,46+,47+,48+,49+,50+,51+,52+,53+,54+,55+,56+,57+,58-,59-,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-/m1/s1
InChI key
DIRLEDPEXJLCIL-JCWBWLHSSA-N
일반 설명
애플리케이션
Succinyl-β-cyclodextrin and carboxymethyl-β-cyclodextrin are used as chiral selective agents in capillary electrophoresis for the separation of di- and tri-peptide enantiomers and catechin enantiomers. Succinyl-β-cyclodextrin is used to optimize analysis of PNA-DNA duplexes with diethylthiadicarbocyanine dye.
- Acylamine fungicides in commercial agrochemical formulations by electrokinetic chromatography (EKC).
- Catechin isomers in human biological samples and antihistamines in pharmaceutical preparations by CE.
기타 정보
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.