추천 제품
분석
≥98.0% (HPLC)
광학 활성
[α]/D +23.0±2°, 24 hr, c = 0.5% in H2O
저장 온도
room temp
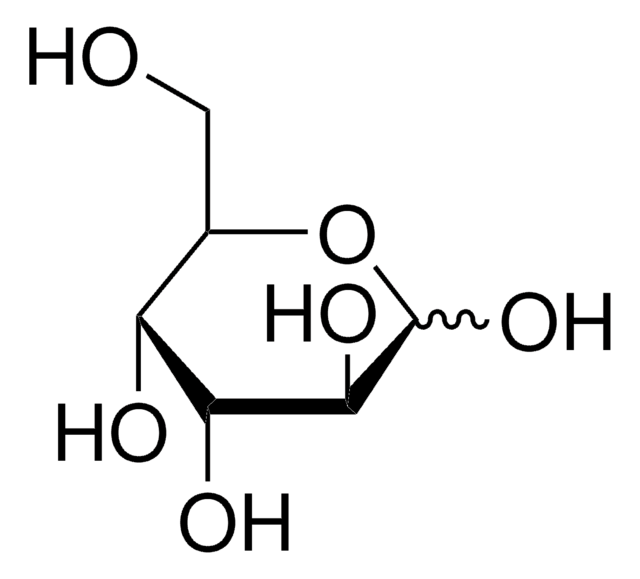
SMILES string
OC[C@@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3+,4-,5-,6?/m0/s1
InChI key
WQZGKKKJIJFFOK-QRXFDPRISA-N
애플리케이션
L-(+)-Gulose, a rare aldohexose sugar C-3 epimer of galactose, is used in the development of various drugs and to glycosylate alginates. L-(+)-Gulose is a substrate of GDP-mannose 3′,5′-epimerase in plants and may be a precursor of L-ascorbic acid in plants.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Daniele Castagnolo et al.
Carbohydrate research, 344(11), 1285-1288 (2009-06-09)
An efficient and stereoselective synthesis of D,L-gulose was described. The key step of the synthetic route is represented by a multicomponent enyne cross metathesis-hetero Diels-Alder reaction which allows the formation of the pyran ring from cheap and commercially available substrates
Jasper Dinkelaar et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(30), 9400-9411 (2008-09-05)
The glycosylation properties of gulopyranosides have been mapped out, and it is shown that gulose has an intrinsic preference for the formation of 1,2-cis-glycosidic bonds. It is postulated that this glycosylation behaviour originates from nucleophilic attack at the oxacarbenium ion
Yunshan Sun et al.
The Journal of organic chemistry, 77(17), 7401-7410 (2012-08-03)
A versatile synthesis of orthogonally protected derivatives of carba-α-D-glucosamine, carba-α-D-mannose, carba-α-D-mannuronic acid, carba-β-L-idosamine, and carba-β-L-gulose from methyl α-D-mannoside is described. Our synthetic strategy utilizes the palladium-promoted Ferrier carbocyclization and persistent butane-2,3-diacetal protection to produce a key chiral cyclohexanone intermediate, from
Saikat Dutta et al.
ACS applied materials & interfaces, 4(3), 1560-1564 (2012-02-18)
Self-assembled TiO(2) nanoparticulate materials with well-defined spherical morphologies were synthesized by using a biopolymer sodium alginate as a template under different synthesis conditions. Powder X-ray diffraction (XRD), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) techniques were used to
Y Zhu et al.
The Journal of organic chemistry, 66(19), 6244-6251 (2001-09-18)
High-resolution (13)C NMR spectra (150 MHz) have been obtained on the complete series of D-aldohexoses (D-allose 1, D-altrose 2, D-galactose 3, D-glucose 4, D-gulose 5, D-idose 6, D-mannose 7, D-talose 8) selectively labeled with (13)C at C1 in order to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.