40674
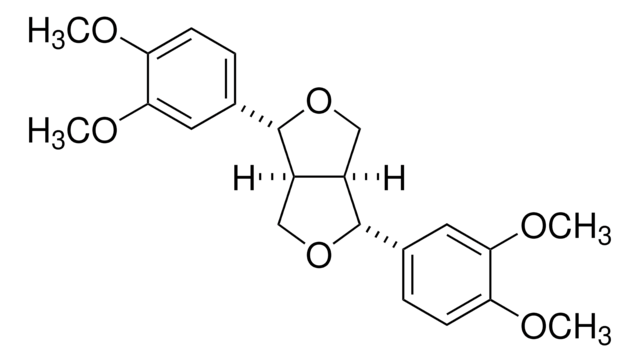
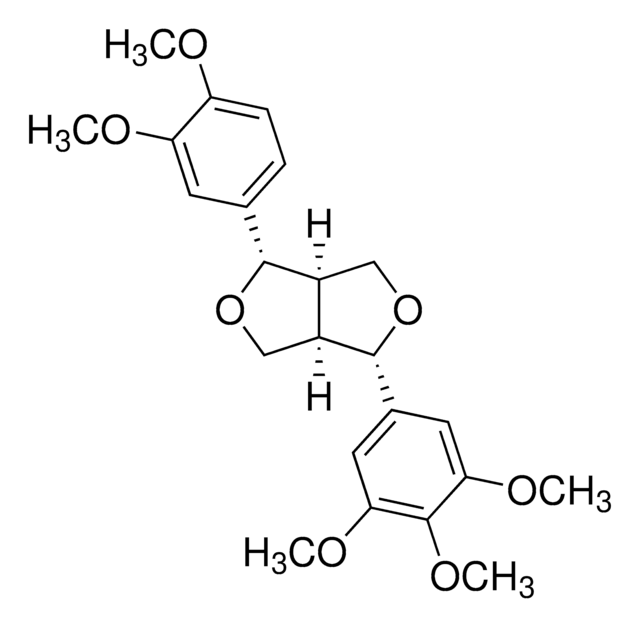
Pinoresinol
≥95.0% (HPLC)
동의어(들):
(+)-Pinoresinol, 4,4′-((1S,3aR,4S,6aR)-Hexahydrofuro[3,4-c]furan-1,4-diyl)bis(2-methoxyphenol), 4,4′-[(1S,3aR,4S,6aR)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2-methoxyphenol)
About This Item
추천 제품
Quality Level
분석
≥95.0% (HPLC)
형태
powder or crystals
응용 분야
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
COc1cc(ccc1O)[C@H]2OC[C@H]3[C@@H]2CO[C@@H]3c4ccc(O)c(OC)c4
InChI
1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19+,20+/m0/s1
InChI key
HGXBRUKMWQGOIE-AFHBHXEDSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- as a reference standard for qualitative and quantitative analysis of lignans from Triticale (X Triticosecale Wittmack) grains using ultra-performance liquid chromatography (UPLC) with photodiode and mass TQD detectors
- as an enterolignan precursor to study its estrogenic activity on the proliferation of human breast cancer MCF-7 cells
- as a reference standard for lignan analysis of Sesamum indicum L. seeds
생화학적/생리학적 작용
포장
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.