추천 제품
Grade
ACS reagent
Quality Level
분석
≥98.0%
양식
crystalline powder
crystalline solid
crystals or chunks
불순물
≤0.02% insolubles
무기 잔류물
≤0.10%
pH
4.5-6.0 (25 °C, 114.1 g/L)
mp
131-135 °C (lit.)
양이온 미량물
heavy metals: ≤5 ppm
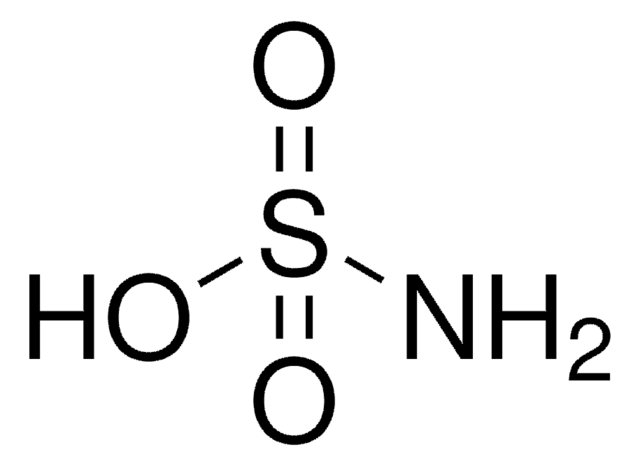
SMILES string
N.NS(O)(=O)=O
InChI
1S/H3NO3S.H3N/c1-5(2,3)4;/h(H3,1,2,3,4);1H3
InChI key
GEHMBYLTCISYNY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Ammonium sulfamate can be used as a catalyst to synthesize arylidene derivatives via microwave-enhanced Knoevenagel condensation.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
S Aleryani et al.
The Journal of biological chemistry, 273(11), 6041-6045 (1998-04-16)
Incubation of S-nitrosocysteine or S-nitrosoglutathione (5-100 M) in the presence of a generator of superoxide (xanthine/xanthine oxidase) resulted in a time-dependent decomposition of S-nitrosothiols and accumulation of nitrite/nitrate in reaction mixtures. Quantitatively, the amounts of nitrite/nitrate represented >90% of nitrosonium
The Chemical Behavior of Low Valence Sulfur Compounds. III. Production of Ammonium Sulfamate by the Oxidation of Ammonium Thiosulfate.
Naito K, et al.
Bulletin of the Chemical Society of Japan, 43(5), 1365-1372 (1970)
Synthesis of Ammonium Dinitramide by Nitration of Potassium and Ammonium Sulfamate. The Effect of Sulfamate Conterion on ADN Purity.
Nazeri GH, et al.
Iranian Journal of Chemistry and Chemical Engineering, 27(!) (2008)
Microwave-enhanced Knoevenagel condensation catalyzed by NH2SO3NH4
Liu C, et al.
Molecular Catalysis, 258, 367-370 (2006)
Ammonium Sulfamate as Substitute for Lead Peroxide in Microdetermination of Carbon and Hydrogen.
Hussey AS, et al.
Analytical Chemistry, 27(2), 280-281 (1955)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.