모든 사진(4)
About This Item
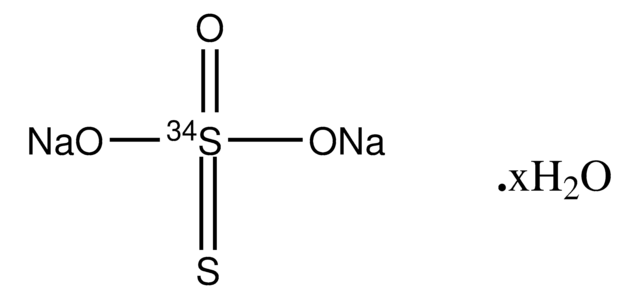
Linear Formula:
Na2S2O3 · 5H2O
CAS Number:
Molecular Weight:
248.18
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.21
분석:
≥99.5%
Grade:
ACS reagent
양식:
crystalline powder
crystals
pellets
crystals
pellets
solubility:
water: soluble(lit.)
추천 제품
Grade
ACS reagent
Quality Level
Agency
suitable for EPA 1621
suitable for ISO 25101
suitable for SM 2310
suitable for SM 2320
suitable for SM 4500 - NH3
분석
≥99.5%
양식
crystalline powder
crystals
pellets
불순물
≤0.002% N compounds
≤0.005% insolubles
pH
6.0-8.4 (25 °C, 5%)
solubility
water: soluble(lit.)
음이온 미량물
S2-: passes test
SO42- and SO32-: ≤0.1%
양이온 미량물
N: ≤0.002%
SMILES string
O.O.O.O.O.[Na+].[Na+].[O-]S([O-])(=O)=S
InChI
1S/2Na.H2O3S2.5H2O/c;;1-5(2,3)4;;;;;/h;;(H2,1,2,3,4);5*1H2/q2*+1;;;;;;/p-2
InChI key
PODWXQQNRWNDGD-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Sodium thiosulfate pentahydrate is a hydrated form of sodium thiosulfate. It is used as a sulfur source for the preparation of various organosulfur compounds.
애플리케이션
Applied in a widely useful, thiosulfate-assisted, synthesis of a variety of diamine-CuCN complexes.
Sodium thiosulfate pentahydrate can be used as a sulfur source to synthesize:
It can also be used in the fabrication of microencapsulated phase change materials for thermal energy storage applications.
- Thioester derivatives via one-pot two-step reactions with organic halides and aryl anhydrides.
- Sulfur nanoparticles using F. benghalensis leaf extract which acts as a reducing and capping agent.
- Unsymmetrical heteroaryl thioethers via multicomponent reaction with heteroaryl chlorides and alcohols.
It can also be used in the fabrication of microencapsulated phase change materials for thermal energy storage applications.
특징 및 장점
Na2S2O3 5H2O is a low toxic and odorless reagent that can be easily used in large-scale operations.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Nuclear magnetic resonance in sodium thiosulfate pentahydrate.
Murty CRK and El Saffar ZM.
Acta Crystallographica, 15(6), 536-538 (1962)
Sodium thiosulfate pentahydrate: a redetermination by neutron diffraction.
Lisensky GC and Levy HA.
Acta Crystallographica Section B, Structural Science, Crystal Engineering and Materials, 34(6), 1975-1977 (1978)
Fingerprint imaging by scanning electrochemical microscopy.
Zhang M and Girault HH.
Electrochemical Communications, 9(7), 1778-1782 (2007)
Crystal Structures of a Series of Complexes Produced by Reaction of Copper(I) Cyanide with Diamines.
Fred B. Stocker et al.
Inorganic chemistry, 38(5), 984-991 (2001-10-24)
A new synthetic procedure developed recently in our laboratories has made possible the synthesis of variety of new complexes of CuCN with diamines. Synthesis was effected by adding the ligand to a solution of CuCN in aqueous sodium thiosulfate. This
Michael C Reade et al.
Emergency medicine Australasia : EMA, 24(3), 225-238 (2012-06-08)
Cyanide poisoning is uncommon, but generates interest because of the presumed utility of an antidote immediately available in those areas with a high risk of cyanide exposure. As part of its regular review of guidelines, the Australian Resuscitation Council conducted
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.