추천 제품
생물학적 소스
synthetic
Grade
pharmaceutical primary standard
Agency
EP
API family
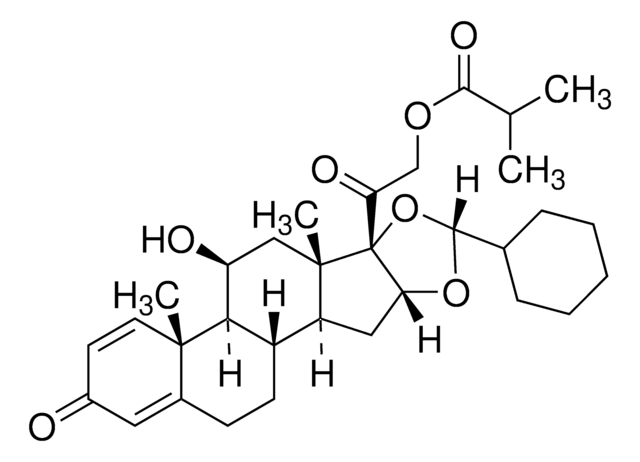
ciclesonide
형태
powder
포장
pkg of 10 mg
제조업체/상표
EDQM
저장 조건
protect from light
solubility
water: <0.1 g/L
응용 분야
pharmaceutical (small molecule)
형식
neat
배송 상태
ambient
저장 온도
2-8°C
InChI
1S/C28H38O6/c1-26-11-10-18(30)12-17(26)8-9-19-20-13-23-28(22(32)15-29,27(20,2)14-21(31)24(19)26)34-25(33-23)16-6-4-3-5-7-16/h10-12,16,19-21,23-25,29,31H,3-9,13-15H2,1-2H3/t19-,20-,21-,23+,24+,25+,26-,27-,28+/m0/s1
InChI key
OXPLANUPKBHPMS-ZXBNPROVSA-N
일반 설명
Ciclesonide impurity B is an impurity of ciclesonide, a new-generation, non-halogenated glucocorticoid.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Ciclesonide impurity B is used as an EP reference standard to quantify the analyte in pharmaceutical formulations by liquid chromatography (LC) technique.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
가장 최신 버전 중 하나를 선택하세요:
New Drugs for Asthma, Allergy and COPD, 42, 42-54 (2001)
Rethinking Cleaning Validation for API Manufacturing
Zhang C, et al.
Pharmaceutical Technology, 42, 42-54 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.