추천 제품
Grade
pharmaceutical primary standard
API family
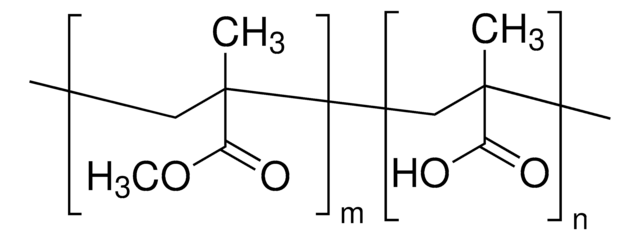
copolymers, butylated methacrylate copolymer
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
InChI
1S/C8H15NO2.C8H14O2.C5H8O2/c1-7(2)8(10)11-6-5-9(3)4;1-4-5-6-10-8(9)7(2)3;1-4(2)5(6)7-3/h1,5-6H2,2-4H3;2,4-6H2,1,3H3;1H2,2-3H3
InChI key
NEDGUIRITORSKL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Basic butylated methacrylate copolyme EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
가장 최신 버전 중 하나를 선택하세요:
Punit P Shah et al.
AAPS PharmSciTech, 9(2), 377-389 (2008-04-24)
The objective of the present investigation was to reduce the bitterness with improved dissolution, in acidic medium (pH 1.2), of mefloquine hydrochloride (MFL). Microparticles were prepared by coacervation method using Eudragit E (EE) as polymer and sodium hydroxide as precipitant.
Alvaro Goyanes et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 79(3), 658-663 (2011-09-29)
This study investigates the extrusion-spheronization performance of some mixtures of co-processed microcrystalline cellulose and Eudragit® E (as excipients) and sorbitol (as soluble filler-disintegrant). Attention is focused on the dissolution rate of low water solubility drugs (hydrochlorothiazide is used as a
K Małolepsza-Jarmołowska et al.
Die Pharmazie, 58(4), 260-262 (2003-05-17)
Intravaginal tablets based on hydrophilic methylcellulose and containing lactic acid complexed with Eudragit E-100 undergo deformation under standard conditions. The high flow--limit of gel originating from the tablets as well as its dynamic viscosity should enable durability of this dosage
Jinhe Li et al.
AAPS PharmSciTech, 3(4), E33-E33 (2003-08-15)
United States Pharmacopeia dissolution apparatus II (paddle) and III (reciprocating cylinder) coupled with automatic sampling devices and software were used to develop a testing procedure for acquiring release profiles of colon-specific drug delivery system (CODES) drug formulations in multi-pH media
L S Ranzani et al.
Drug development and industrial pharmacy, 37(6), 694-701 (2011-01-14)
The aim of the present work was to investigate in vitro dissolution properties of three binary solid solutions prepared by a hot-melt extrusion (HME) process with vinyl pirrolidone--vinyl acetate copolymer (Kollidon VA 64), ethyl acrylate, methyl methacrylate polymer (Eudragit E)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.