모든 사진(1)
About This Item
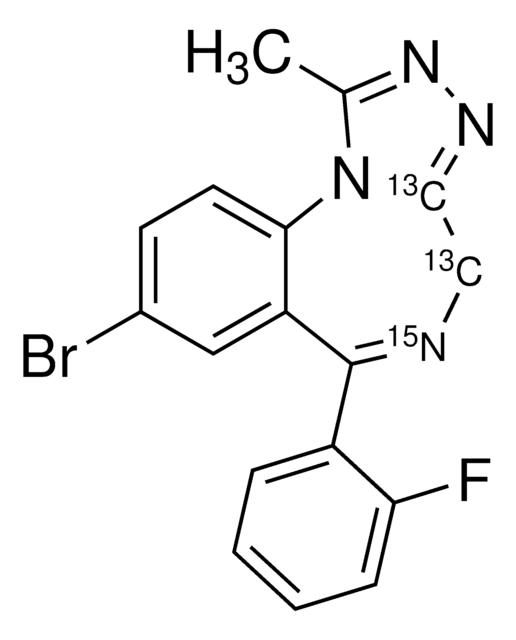
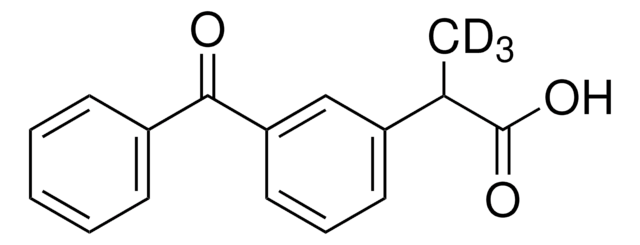
실험식(Hill 표기법):
C15H10BrClN4S
CAS Number:
Molecular Weight:
393.69
UNSPSC 코드:
41116107
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
brotizolam
제조업체/상표
EDQM
drug control
psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
[s]1c2c(cc1Br)C(=NCc4[n]2c(nn4)C)c3c(cccc3)Cl
InChI
1S/C15H10BrClN4S/c1-8-19-20-13-7-18-14(9-4-2-3-5-11(9)17)10-6-12(16)22-15(10)21(8)13/h2-6H,7H2,1H3
InChI key
UMSGKTJDUHERQW-UHFFFAOYSA-N
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Brotizolam EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
가장 최신 버전 중 하나를 선택하세요:
Akshay Anand et al.
Behavioural brain research, 182(1), 12-20 (2007-06-15)
Benzodiazepines such as diazepam, lorazepam, are reported to produce anterograde amnesia but these do not affect the retrieval mechanism. Triazodiazepines such as alprazolam, triazolam and brotizolam produce both anterograde and retrograde amnesia. Because benzodiazepine receptor antagonists are known to reverse
Atsushi Takiguchi et al.
Journal of pharmacological sciences, 101(4), 325-328 (2006-08-08)
Triazolam caused no significant increase in the total error at 0.05 and 0.1 mg/kg. However, at 0.2 mg/kg, it caused a significant increase in total error. Almost the same findings were observed with brotizolam and rilmazafone. That is, at 0.2
Naoko Tanaka et al.
Soudni lekarstvi, 56(1), 5-6 (2011-03-19)
A case of drowning involving brotizolam, flunitrazepam and ethanol ingestion was presented. Quantitative toxicological analysis showed that the concentrations of brotizolam, 7-aminoflunitrazepam (a metabolite of flunitrazepam) and ethanol in the femoral blood were 0.025 microg/ml, 0.094 microg/ml and 0.29 mg/ml
Yoshinori Sugimoto et al.
Bioorganic & medicinal chemistry letters, 23(12), 3515-3518 (2013-05-15)
When the drug product of brotizolam (1) is decomposed according to the interview form and patent, hydrolysate (2) is obtained, but its physicochemical data are still missing. To elucidate the structure based on a spectroscopic approach, the above-mentioned degradation product
Yukihiro Ujiie et al.
Therapeutic drug monitoring, 28(3), 299-302 (2006-06-17)
The purpose of the present study was to examine the effects of rifampicin on the single oral dose pharmacokinetics and pharmacodynamics of brotizolam. Thirteen healthy male volunteers received rifampicin 450 mg/day, or matched placebo, for 7 days in a double-blind
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.