추천 제품
Grade
pharmaceutical primary standard
API family
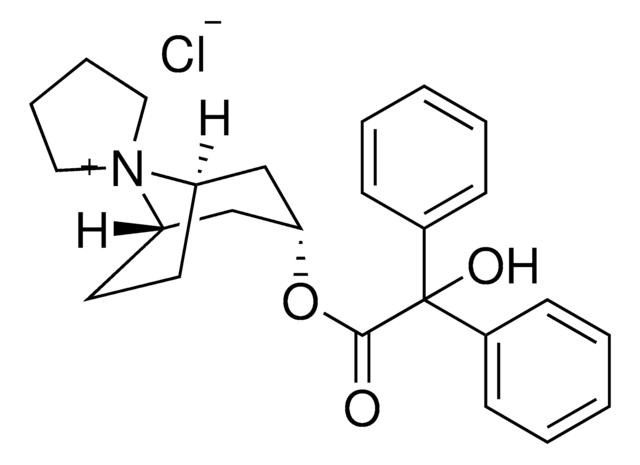
trospium
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
[Cl-].[N+]21(C3CCC2CC(C3)OC(=O)C(O)(c5ccccc5)c4ccccc4)CCCC1
InChI
1S/C25H30NO3.ClH/c27-24(25(28,19-9-3-1-4-10-19)20-11-5-2-6-12-20)29-23-17-21-13-14-22(18-23)26(21)15-7-8-16-26;/h1-6,9-12,21-23,28H,7-8,13-18H2;1H/q+1;/p-1
InChI key
RVCSYOQWLPPAOA-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Trospium chloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
가장 최신 버전 중 하나를 선택하세요:
Anthony G Visco et al.
The New England journal of medicine, 367(19), 1803-1813 (2012-10-06)
Anticholinergic medications and onabotulinumtoxinA are used to treat urgency urinary incontinence, but data directly comparing the two types of therapy are needed. We performed a double-blind, double-placebo-controlled, randomized trial involving women with idiopathic urgency urinary incontinence who had five or
Scott A MacDiarmid et al.
Urology, 77(1), 24-29 (2010-10-26)
This study used pooled data from 2 large, phase III, double-blind, randomized, placebo-controlled studies for a subgroup analysis of the safety and efficacy of trospium chloride extended-release (XR) in men with overactive bladder (OAB). A subgroup analysis was performed on
Martina Urbanova et al.
Journal of pharmaceutical sciences, 102(4), 1235-1248 (2013-01-30)
Analysis of C cross-polarization magic angle spinning (CP/MAS) nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC), Fourier transform infrared (FTIR), and X-ray powder diffraction data of trospium chloride (TCl) products crystallized from different mixtures of water-ethanol [φ(EtOH) = 0.5-1.0] at
R Zhang et al.
Arzneimittel-Forschung, 62(5), 247-251 (2012-03-03)
The study aimed to compare and evaluate the bioequivalence of a new generic preparation of trospium chloride (CAS NO:10405-02-4) capsule (20 mg, test) and the available import tablet (20 mg , reference) for the requirement of state regulatory criteria in
V V Danilov et al.
Urologiia (Moscow, Russia : 1999), (4)(4), 15-20 (2010-10-26)
The analysis of 58 cases of overactive bladder has shown that detrusor activity is not linked with clinical symptoms but is caused by supra segmentary lesion of the nervous system. The clinical picture of overactive bladder fits the proposed neurophysiological
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.