83916
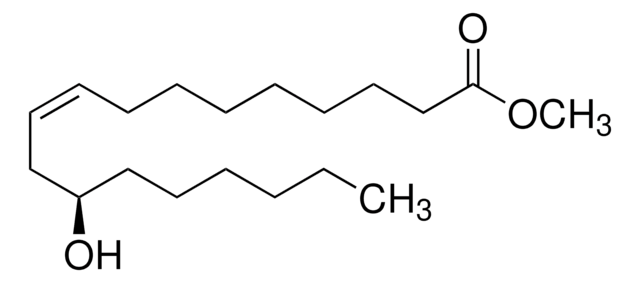
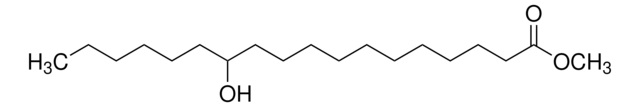
Methyl ricinoleate
analytical standard
동의어(들):
(R)-12-Hydroxy-cis-9-octadecenoic acid methyl ester, Methyl 12-hydroxyoleate, Ricinoleic acid methyl ester
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C19H36O3
CAS Number:
Molecular Weight:
312.49
Beilstein:
6132055
EC Number:
MDL number:
UNSPSC 코드:
85151701
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
analytical standard
Quality Level
분석
≥99.0% (GC)
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
형식
neat
작용기
ester
배송 상태
ambient
저장 온도
−20°C
SMILES string
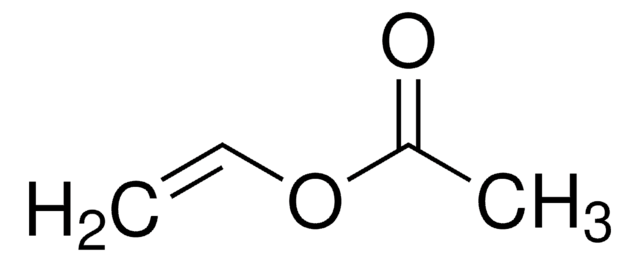
O=C(OC)CCCCCCC/C=C\C[C@H](O)CCCCCC
InChI
1S/C19H36O3/c1-3-4-5-12-15-18(20)16-13-10-8-6-7-9-11-14-17-19(21)22-2/h10,13,18,20H,3-9,11-12,14-17H2,1-2H3/b13-10-/t18-/m1/s1
InChI key
XKGDWZQXVZSXAO-ADYSOMBNSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Methyl ricinoleate is an enantiomerically renewable compound commercially obtained by the transesterification from castor oil, commonly used in cosmetics, plasticizers, lubricating oils and fine-chemical industries.
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Optimization of methyl ricinoleate synthesis with ionic liquids as catalysts using the response surface methodology.
Xu W, et al.
Chemical Engineering Journal, 275(11), 63-70 (2015)
Synthesis of Enantiomerically Pure 2, 3, 4, 6-Tetrasubstituted Tetrahydropyrans by Prins-Type Cyclization of Methyl Ricinoleate and Aldehydes.
Biermann U, et al.
European Journal of Organic Chemistry, 2006(11), 2631-2637 (2006)
Y Waché et al.
Applied and environmental microbiology, 67(12), 5700-5704 (2001-11-28)
Some microorganisms can transform methyl ricinoleate into gamma-decalactone, a valuable aroma compound, but yields of the bioconversion are low due to (i) incomplete conversion of ricinoleate (C(18)) to the C(10) precursor of gamma-decalactone, (ii) accumulation of other lactones (3-hydroxy-gamma-decalactone and
A Endrizzi et al.
Journal of basic microbiology, 35(5), 285-292 (1995-01-01)
The capacity of several strains of yeasts to do the bioconversion of methyl ricinoleate into gamma-decalactone, was studied in a medium containing this methylic ester of fatty acid as sole carbon source. Amongst the strains which are able to do
Y Waché et al.
Letters in applied microbiology, 30(3), 183-187 (2000-04-04)
Size of methyl ricinoleate droplets during biotransformation into gamma-decalactone by Yarrowia lipolytica was measured in both homogenized and non-homogenized media. In non-homogenized but shaken medium, droplets had an average volume surface diameter d32 of 2.5 microm whereas it was 0.7
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.