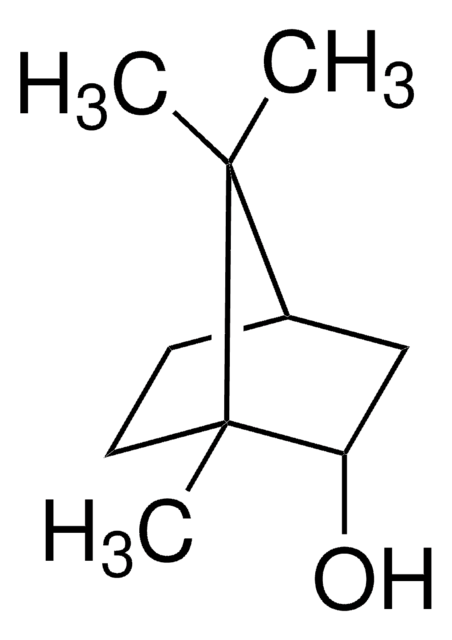
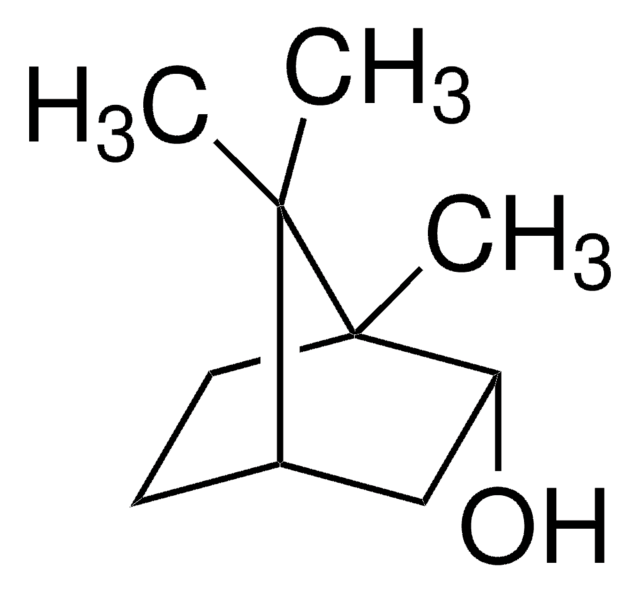
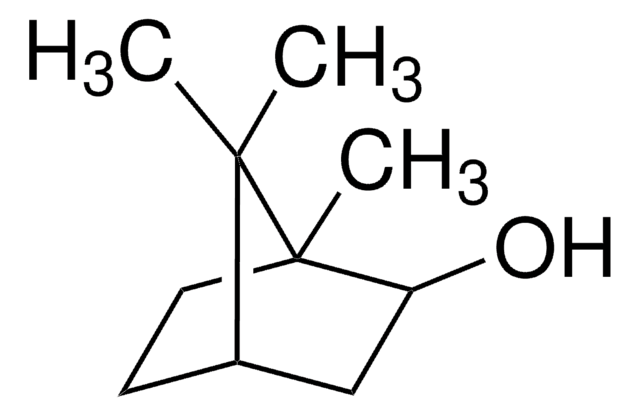
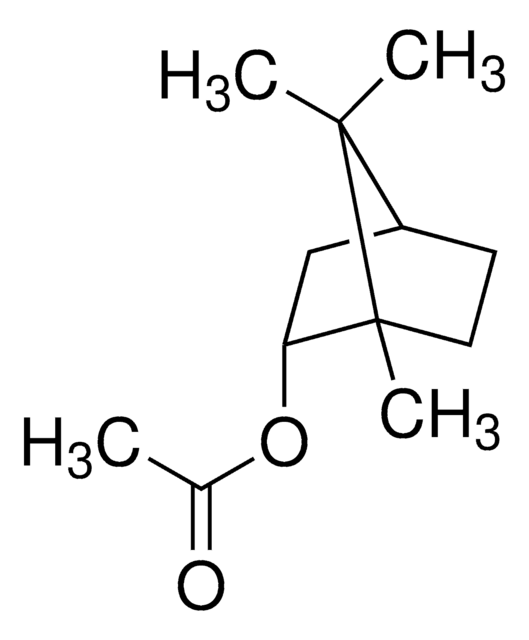
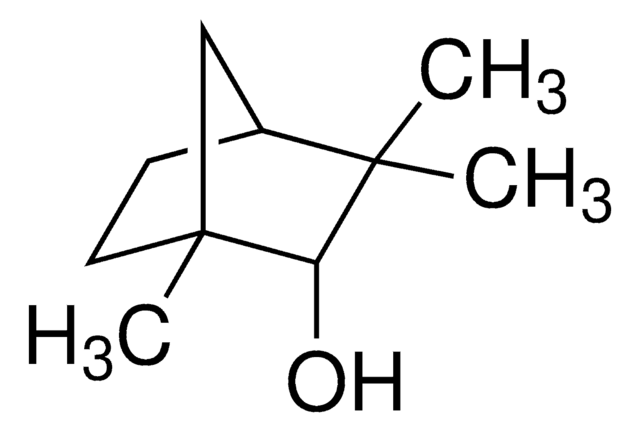
추천 제품
Grade
analytical standard
Quality Level
분석
≥99.0% (sum of enantiomers, GC)
광학 활성
[α]20/D +11.0±0.5°, c = 10% in ethanol
유통기한
limited shelf life, expiry date on the label
기술
HPLC: suitable
gas chromatography (GC): suitable
bp
201-202 °C (lit.)
mp
43-46 °C
형식
neat
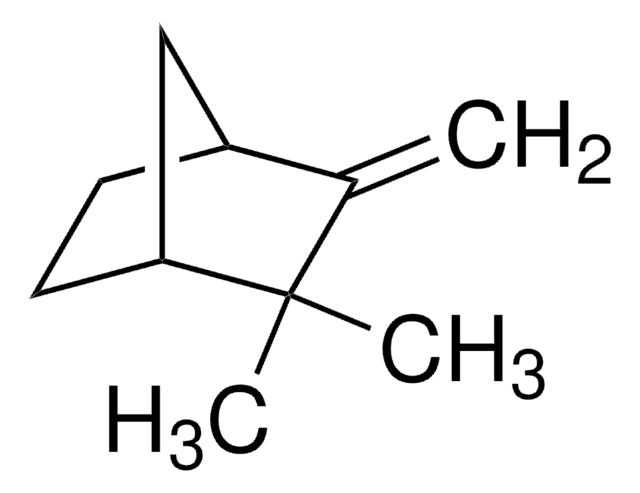
SMILES string
CC1(C)[C@H]2CC[C@](C)(C2)[C@H]1O
InChI
1S/C10H18O/c1-9(2)7-4-5-10(3,6-7)8(9)11/h7-8,11H,4-6H2,1-3H3/t7-,8-,10+/m0/s1
InChI key
IAIHUHQCLTYTSF-OYNCUSHFSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
기타 정보
Chiral building block
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
165.2 °F - closed cup
Flash Point (°C)
74 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
A.A. van der Zeijden et al.
Synthesis, 847-847 (1996)
Y. Yuasa et al.
Tetrahedron, 48, 3473-3473 (1992)
M Miyazawa et al.
Xenobiotica; the fate of foreign compounds in biological systems, 37(9), 943-953 (2007-11-10)
The metabolism of (+)-fenchol was investigated in vitro using liver microsomes of rats and humans and recombinant cytochrome P450 (P450 or CYP) enzymes in insect cells in which human/rat P450 and NADPH-P450 reductase cDNAs had been introduced. The biotransformation of
Sergio Abbate et al.
Chirality, 21 Suppl 1, E242-E252 (2009-11-21)
The first well documented experiments of Near Infrared Vibrational Circular Dichroism (NIR-VCD) were performed around 1975. We review the thirty year history of NIR-VCD, encompassing both instrumental development and theoretical/computational methods that allow interpretation of experimental spectra, harvesting useful structural
D M Satterwhite et al.
The Journal of biological chemistry, 260(26), 13901-13908 (1985-11-15)
The conversion of geranyl pyrophosphate to (-)-endo-fenchol is considered to proceed by the initial isomerization of the substrate to (-)-(3R)-linalyl pyrophosphate and the subsequent cyclization of this bound intermediate. To test this stereochemical scheme, phosphatase-free preparations of (-)-endo-fenchol cyclase from
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.