00848
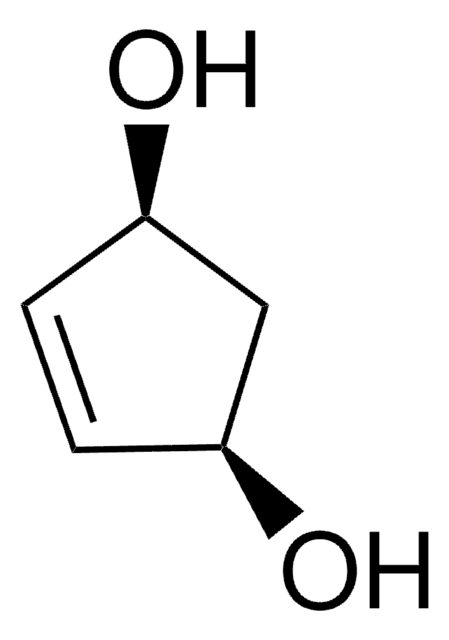
(1R,4S)-cis-4-Acetoxy-2-cyclopenten-1-ol
≥98.0% (sum of enantiomers, GC)
동의어(들):
(1S,4R)-cis-4-Hydroxy-2-cyclopentenyl acetate
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C7H10O3
CAS Number:
Molecular Weight:
142.15
Beilstein:
4350633
MDL number:
UNSPSC 코드:
12352108
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
≥98.0% (sum of enantiomers, GC)
광학 활성
[α]20/D −67±2°, c = 2.3% in chloroform
mp
49-51 °C
작용기
ester
hydroxyl
SMILES string
CC(=O)O[C@H]1C[C@@H](O)C=C1
InChI
1S/C7H10O3/c1-5(8)10-7-3-2-6(9)4-7/h2-3,6-7,9H,4H2,1H3/t6-,7+/m0/s1
InChI key
IJDYOKVVRXZCFD-NKWVEPMBSA-N
애플리케이션
(1R,4S)-cis-4-Acetoxy-2-cyclopenten-1-ol can be used as a reactant in the total synthesis of Bartlett′s brefeldin intermediate, spinosyn A analogs, (+)-7-deaza-5′-noraristeromycin, (±) strychnine and the Wieland-Gumlich aldehyde.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
A new approach to (+)-brefeldin A via a nickel-catalyzed coupling reaction of cyclopentenyl acetate and lithium 2-furylborate
Kobayashi Y, et al.
Tetrahedron Letters, 37(34), 6125-6128 (1996)
Asymmetric Total Syntheses of (-)-and (+)-Strychnine and the Wieland-Gumlich Aldehyde
Knight SD, et al.
Journal of the American Chemical Society, 117(21), 5776-5788 (1995)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.