추천 제품
생물학적 소스
mouse
Quality Level
항체 형태
ascites fluid
항체 생산 유형
primary antibodies
클론
A1, monoclonal
A3, monoclonal
종 반응성
human
제조업체/상표
Chemicon®
기술
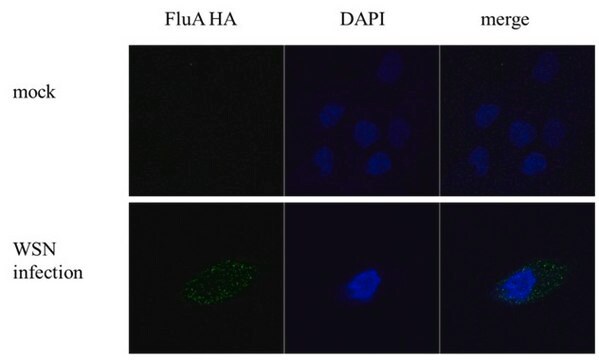
immunofluorescence: suitable
동형
IgG1 (A3)
IgG2a (A1)
배송 상태
wet ice
특이성
All subtypes of Influenza A (H1N1, H2N2 and H3N2 and H5N1). Specific for the nucleoprotein antigen. No cross-reactivity seen to Influenza B, Parainfluenza 1, 2 or Adenovirus or RSV.
면역원
Influenza A blend.
애플리케이션
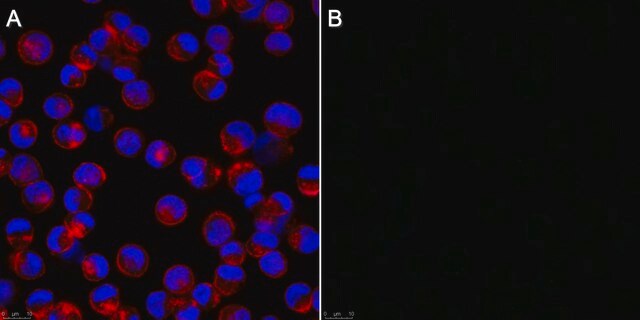
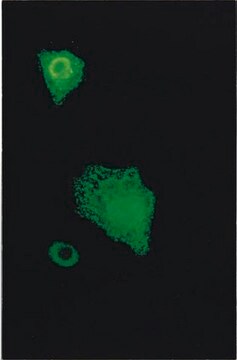
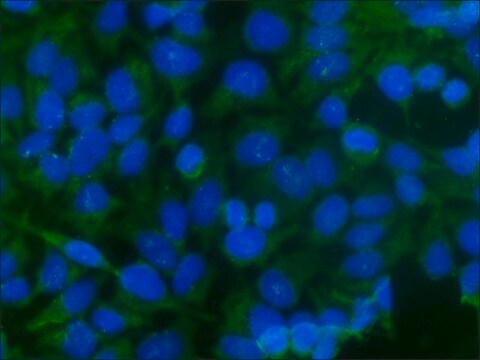
Indirect immunofluorescence at 1:100-1:1000.
Optimal working dilutions must be determined by end user.
Optimal working dilutions must be determined by end user.
Research Category
Infectious Diseases
Infectious Diseases
Research Sub Category
Infectious Diseases - Viral
Infectious Diseases - Viral
This Anti-Influenza A Antibody, nucleoprotein, clones A1, A3 Blend is validated for use in IF for the detection of Influenza A.
물리적 형태
Ascites fluid containing no preservatives.
Unpurified
저장 및 안정성
Maintain for 1 year at -20°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
분석 메모
Control
Influenza Control Slides, Catalogue Number 5010-5
Influenza Control Slides, Catalogue Number 5010-5
기타 정보
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
법적 정보
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
면책조항
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Recipients of vaccine against the 1976 "swine flu" have enhanced neutralization responses to the 2009 novel H1N1 influenza virus.
McCullers, JA; Van De Velde, LA; Allison, KJ; Branum, KC; Webby, RJ; Flynn, PM
Clinical Infectious Diseases null
Higher level of replication efficiency of 2009 (H1N1) pandemic influenza virus than those of seasonal and avian strains: kinetics from epithelial cell culture and computational modeling.
Mitchell, H; Levin, D; Forrest, S; Beauchemin, CA; Tipper, J; Knight, J; Donart et al.
Journal of virology null
Maren Eggers et al.
Infectious diseases and therapy, 7(2), 249-259 (2018-04-11)
Recent virus epidemics and rising antibiotic resistance highlight the importance of hygiene measures to prevent and control outbreaks. We investigated the in vitro bactericidal and virucidal efficacy of povidone-iodine (PVP-I) 7% gargle/mouthwash at defined dilution against oral and respiratory tract
Tissue tropism of a Thailand strain of high-pathogenicity avian influenza virus (H5N1) in tissues of naturally infected native chickens (Gallus gallus), Japanese quail (Coturnix coturnix japonica) and ducks (Anas spp.).
Chongmas Antarasena, Rungtiva Sirimujalin, Porntip Prommuang, Stuart D Blacksell et al.
Avian Pathology : Journal of the W.V.P.A null
Rapidly produced SAM(?) vaccine against H7N9 influenza is immunogenic in mice.
Hekele, A; Bertholet, S; Archer, J; Gibson, DG; Palladino, G; Brito, LA; Otten et al.
Emerging microbes & infections null
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.