528116
PI 3-Kα Inhibitor VIII
The PI 3-Kα Inhibitor VIII controls the biological activity of PI 3-Kα. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
동의어(들):
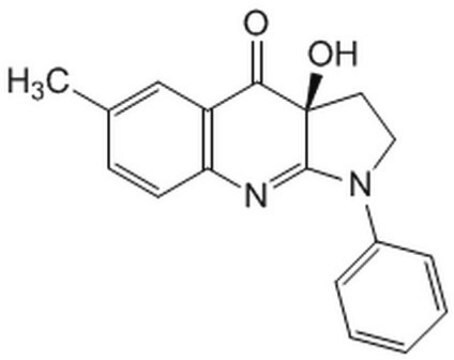
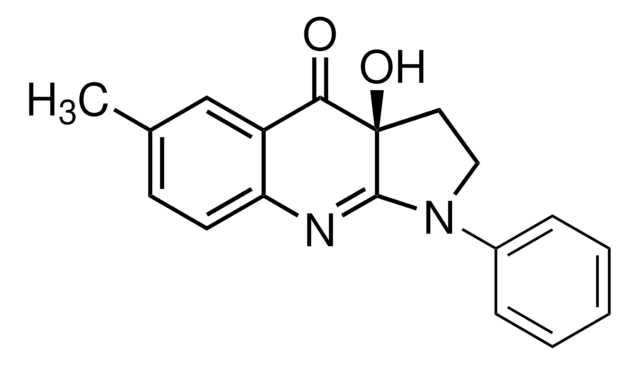
PI 3-Kα Inhibitor VIII, N-((1E)-(6-Bromoimidazo[1,2-a]pyridin-3-yl)methylene)-Nʹ-methyl-Nʹʹ-(2-methyl-5-nitrobenzene)sulfonohydrazide, HCl, PIK-75, PI 3-K Inhibitor VIII
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C16H14BrN5O4S · 2H2O · xHCl
CAS Number:
Molecular Weight:
488.31 (free base basis)
UNSPSC 코드:
12352200
NACRES:
NA.28
추천 제품
Quality Level
분석
≥95% (HPLC)
형태
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
desiccated (hygroscopic)
protect from light
색상
pale yellow
solubility
DMSO: 10 mg/mL
배송 상태
ambient
저장 온도
2-8°C
InChI
1S/C16H14BrN5O4S.ClH/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16;/h3-10H,1-2H3;1H/b19-9+;
InChI key
VOUDEIAYNKZQKM-MYHMWQFYSA-N
일반 설명
A cell-permeable imidazopyridine compound that acts as a highly potent and ATP-competitive DNA-PK and p110α-selective PI3-K inhibitor (IC50 = 0.3, 40, 100, and 850 nM for p110α, p110γ, PI 3-K C2β, and p110β, respectively). Shown to effectively block cellular PI3-K/Akt signaling and inhibit tumor growth both in vitro (IC50 ≤58 nM in MCF7, MCF7 ADR-res, HeLa, A375, and A549 cultures) and in mice in vivo (62% inhibition of HeLa xenograft in 2 weeks, 50 mg/kg/day, i.p.).
포장
Packaged under inert gas
경고
Toxicity: Regulatory Review (Z)
재구성
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
기타 정보
Kim, S., et al. 2007. Blood110, 4206.
Hayakawa, M., et al. 2007. Bioorg. Med. Chem.15, 5837.
Knight, Z.A., et al. 2007. Cell125, 733.
Hayakawa, M., et al. 2007. Bioorg. Med. Chem.15, 5837.
Knight, Z.A., et al. 2007. Cell125, 733.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Soochong Kim et al.
Blood, 110(13), 4206-4213 (2007-09-11)
Platelets release insulin-like growth factor-1 (IGF-1) from alpha granules upon activation. We have investigated the regulation of IGF-1 in G(i)-dependent pathways leading to Akt activation and the role of IGF-1 in platelet activation. IGF-1 alone failed to induce platelet aggregation
Masahiko Hayakawa et al.
Bioorganic & medicinal chemistry, 15(17), 5837-5844 (2007-07-03)
We have previously reported the imidazo[1,2-a]pyridine derivative 4 as a novel p110alpha inhibitor; however, although 4 is a potent inhibitor of p110alpha enzymatic activity and tumor cell proliferation in vitro, it is unstable in solution and ineffective in vivo. To
Zachary A Knight et al.
Cell, 125(4), 733-747 (2006-05-02)
Phosphoinositide 3-kinases (PI3-Ks) are an important emerging class of drug targets, but the unique roles of PI3-K isoforms remain poorly defined. We describe here an approach to pharmacologically interrogate the PI3-K family. A chemically diverse panel of PI3-K inhibitors was
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.