375240
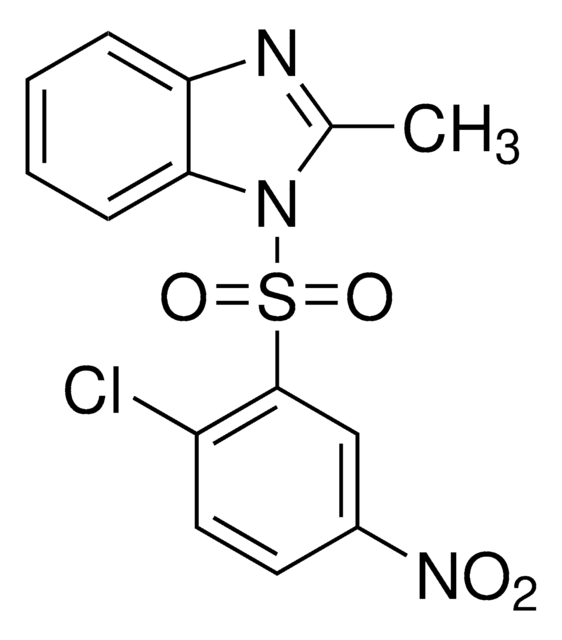
HNF4 Antagonist, BI6015
The HNF4 Antagonist, BI6, also referenced under CAS 93987-29-2, controls the biological activity of HNF4.
동의어(들):
HNF4 Antagonist, BI6015, 2-Methyl-1-(2-methyl-5-nitrophenylsulfonyl)-1H-benzo[d]imidazole, Hepatocyte Nuclear Factor4 Antagonist
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
실험식(Hill 표기법):
C15H13N3O4S
CAS Number:
Molecular Weight:
331.35
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.32
추천 제품
Quality Level
분석
≥99% (HPLC)
양식
powder
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
protect from light
색상
beige
solubility
DMSO: 10 mg/mL
배송 상태
ambient
저장 온도
2-8°C
SMILES string
CC1=NC2=C(N1S(C3=CC([N+]([O-])=O)=CC=C3C)(=O)=O)C=CC=C2
InChI
1S/C15H13N3O4S/c1-10-7-8-12(18(19)20)9-15(10)23(21,22)17-11(2)16-13-5-3-4-6-14(13)17/h3-9H,1-2H3
InChI key
ILVCPQPMRPHZSG-UHFFFAOYSA-N
일반 설명
A cell-permeable phenylsulfonylbenzimidazole compound that is shown to dock in the ligand-binding pocket of both HNF4α and HNF4γ via in silico structural analyses and antagonizes HNF4α DNA binding activity (by 93% after 10 µM overnight treatment of HepG2 cells), effectively inhibiting HNF4α-dependent cellular activities, including HNF4α mRNA transcription (by 62% in murine insulinoma MIN6 and 84% in human hepatoma HepG2 cultures after 5 h and 48 h 5 µM inhibitor treatment, respectively) and OTC (omithine transcarbamoylase) promoter transcription (by 85% & >95% in human HNF4α-transfected HepG2 & CV-1 cells, respectively; 48 hr 1 µM treatment). HNF4γ inhibition by BI6015 is also reported to indirectly lead to decreased binding of transactivators, E47 & PDX-1, to insulin promoter in T6PNE cells (48 h 5 µM treatment). Although BI6015 is found to exhibit cancer-selective cytotoxicity toward a panel of 58 human cancer cells and Hep3B-Luc (Effective conc. 1 to 10 µM), but not primary murine hepatocytes, it does cause hepatic steatosis both in vitro (primary murine hepatocytes; 5 µM for 3 days) and in mice in vivo (10 to 30 mg/kg/day for 5 days via i.p.) and is effectively metabolized by liver enzymes, limiting its in vivo efficacy in treating human Hep3B-derived liver tumor in mice. BI6015 also inhibits Human CYP450 2C19 and rat L-type calcium channel (by 94% and 83%, respectively, at 10 µM), but not PPARγ or a panel of 41 receptors/enzymes of human, mouse, and rat origin.
A cell-permeable phenylsulfonylbenzimidazole that is shown to dock in the ligand-binding pocket of both HNF4α and HNF4γ and antagonize HNF4α DNA binding activity in HepG2 cells (by 93%; 10 µM overnight), effectively inhibiting HNF4α-dependent cellular activities (Effective conc. 1 to 5 µM). HNF4γ inhibition by BI6015 can also lead to decreased insulin promoter binding by transactivators E47 & PDX-1 in T6PNE cells (5 µM 48 h). Although BI6015 is found to exhibit cancer-selective cytotoxicity toward a panel of 58 human cancer cells and Hep3B-Luc (Effective conc. 1 to 10 µM), but not primary murine hepatocytes, it does cause hepatic steatosis both in vitro and in mice in vivo, limiting its use in animal studies. BI6015 also inhibits Human CYP450 2C19 and rat L-type calcium channel (by 94% and 83%, respectively, at 10 µM), but not PPARγ or a panel of 41 receptors/enzymes of human, mouse, and rat origin.
생화학적/생리학적 작용
Cell permeable: yes
Primary Target
HNFα & γ
HNFα & γ
Reversible: yes
포장
Packaged under inert gas
경고
Toxicity: Standard Handling (A)
기타 정보
Kiselyuk, A., et al. 2012. Chem. Biol.19, 806.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.