196876
BAY 41-2272
A cell-permeable pyrazolopyridinylpyrimidine compound that acts as a selective and potent stimulator of soluble guanylate cyclase (effective dose ~ 0.1 nM to 100 µM using recombinant soluble guanylate cyclase).
동의어(들):
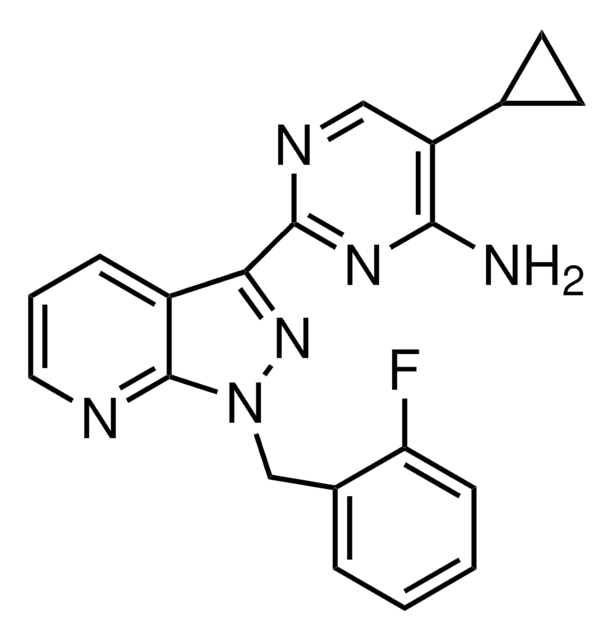
BAY 41-2272, 5-Cyclopropyl-2-[1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-4-ylamine
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H17FN6
CAS Number:
Molecular Weight:
360.39
MDL number:
UNSPSC 코드:
51111800
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
protect from light
색상
white
solubility
DMSO: 10 mg/mL
배송 상태
ambient
저장 온도
2-8°C
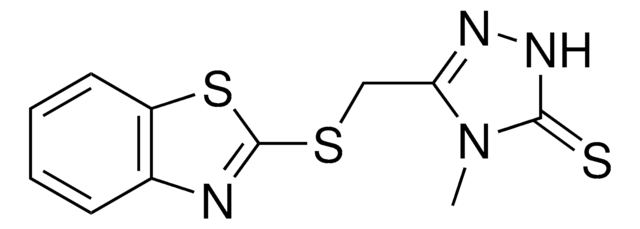
SMILES string
Fc1c(cccc1)C[n]2nc(c5c2nccc5)c3nc(c(cn3)C4CC4)N
InChI
1S/C20H17FN6/c21-16-6-2-1-4-13(16)11-27-20-14(5-3-9-23-20)17(26-27)19-24-10-15(12-7-8-12)18(22)25-19/h1-6,9-10,12H,7-8,11H2,(H2,22,24,25)
InChI key
ATOAHNRJAXSBOR-UHFFFAOYSA-N
일반 설명
A cell-permeable pyrazolopyridinylpyrimidine compound that acts as a selective and potent stimulator of soluble guanylate cyclase (effective dose ~ 0.1 nM to 100 µM using recombinant soluble guanylate cyclase). The mode of activation is NO-independent and appears to be mediated through direct binding to the α1 and α2 subunits. Shown to be effective in treating numerous cardiovascular conditions in animal models. Inhibits phenylephrine-induced constriction of rabbit aortic rings (IC50 = 304 nM) and blocks collagen-induced aggregation of human platelets (IC50 = 36 nM). Does not inhibit phosphodiesterases.
생화학적/생리학적 작용
Cell permeable: yes
Primary Target
Soluble guanylate cyclase
Soluble guanylate cyclase
Product does not compete with ATP.
Reversible: no
Target IC50: 304 nM inhibiting phenylephrine-induced constriction of rabbit aortic rings; 36 nM in blocking collagen-induced aggregation of human platelets
경고
Toxicity: Standard Handling (A)
재구성
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
기타 정보
Boerrigter, G., et al. 2003. Circulation107, 686.
Kalsi, J.S., et al. 2003. J Urol.169, 761.
Koglin, M., et al. 2002. Biochem. Biophys. Res. Commun.292, 1057.
Becker, E.M., et al. 2001. BMC Pharmacol.1, 13.
Stasch, J.P., et al. 2001. Nature410, 212.
Kalsi, J.S., et al. 2003. J Urol.169, 761.
Koglin, M., et al. 2002. Biochem. Biophys. Res. Commun.292, 1057.
Becker, E.M., et al. 2001. BMC Pharmacol.1, 13.
Stasch, J.P., et al. 2001. Nature410, 212.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.