추천 제품
생물학적 소스
synthetic
Quality Level
제품 라인
EMPROVE® EXPERT
형태
powder or crystals
포장
pkg of 1 kg (in PE drum, 2 inner PE liners 1371191000)
pkg of 10 kg (in PE drum, 2 inner PE liners 1371199010)
pkg of 100 g (in PE tub, inner glas bottle 1371190100)
pH
5.5-9.0 (10 g/L in H2O)
solubility
25 g/L
≤70 g/L (in complex feed)
적합성
suitable for manufacturing use (cell culture)
저장 온도
2-8°C
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Modified amino acids are inhouse manufactured amino acid derivatives with specific properties enabling the intensification of cell culture processes.
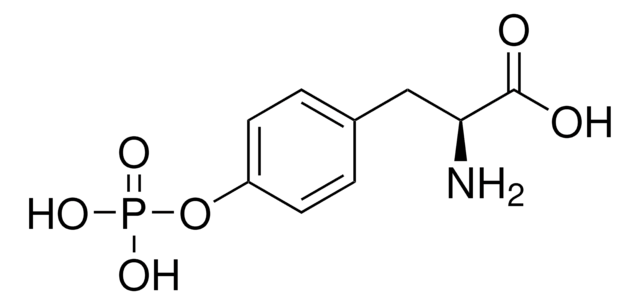
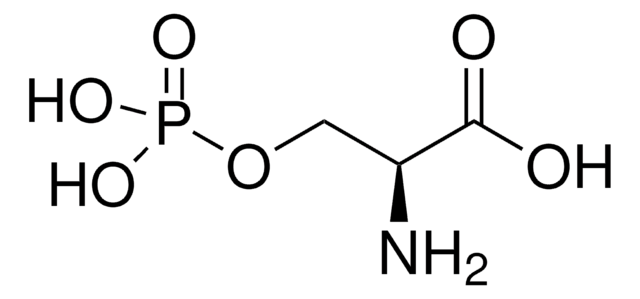
Together with the companion product Sulfo-Cysteine sodium salt, the new modified amino acid Phospho-Tyrosine disodium salt can be used as replacement for tyrosine to generate highly concentrated, neutral pH feeds. Both modified amino acids eliminate the need for alkaline feeds, which are normally applied to ensure solubility and stability of the unmodified amino acids tyrosine and cysteine.
Together with the companion product Sulfo-Cysteine sodium salt, the new modified amino acid Phospho-Tyrosine disodium salt can be used as replacement for tyrosine to generate highly concentrated, neutral pH feeds. Both modified amino acids eliminate the need for alkaline feeds, which are normally applied to ensure solubility and stability of the unmodified amino acids tyrosine and cysteine.
Our SAFC® portfolio of high-quality products for biopharmaceutical processing withstands strict quality control procedures and is produced according to MQ-500 requirements as defined by the M-Clarity program.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of upstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Trust us to deliver supply chain transparency and reliable sourcing around the globe, streamlining your product qualification with best-in-class regulatory support and service.
M-Clarity Program
As part of our EMPROVE® Program, our raw materials are offered with EMPROVE® Dossiers which provide comprehensive, up-to-date documentation to help you navigate regulatory challenges, manage risks, and improve your manufacturing processes.
Our comprehensive portfolio of upstream process chemicals not only provides biopharmaceutical manufacturers with high-quality raw materials for production of classical and novel therapies, but also helps them get to market faster and simplify regulatory challenges. Trust us to deliver supply chain transparency and reliable sourcing around the globe, streamlining your product qualification with best-in-class regulatory support and service.
특징 및 장점
- Reduced complexity in fed-batch process
- High concentrations of modified tyrosine in main feeds at neutral pH
- Enhanced solubility up to 70g/l in complex feed
- Prevention of caustic shocks in the bioreactor due to high pH feeds
- More convenient preparation process with less contamination risks
- Higher feed stability at room temperature
결합
법적 정보
Emprove is a registered trademark of Merck KGaA, Darmstadt, Germany
SAFC is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.