123040
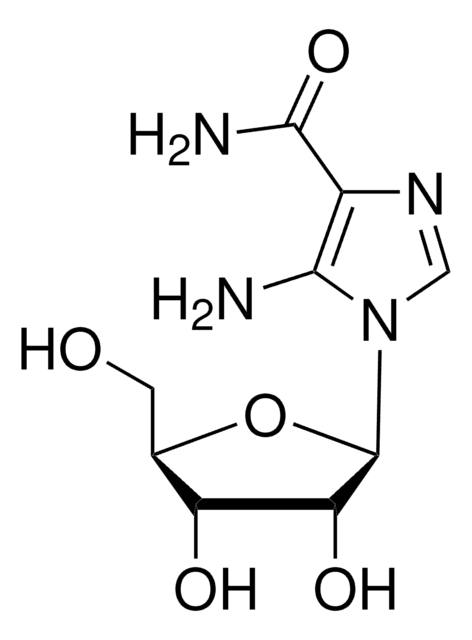
AICA-Riboside
AICA-Riboside, CAS 2627-69-2, is a cell-permeable nucleoside compound whose phosphorylated metabolite activates AMPK and acts as a regulator of de novo purine synthesis.
동의어(들):
AICA-Riboside, Acadesine, AICAr, 5-Aminoimidazole-4-carboxamide-1-β-riboside, Z-Riboside, AMPK Signaling Activator II
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C9H14N4O5
CAS Number:
Molecular Weight:
258.23
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
제조업체/상표
Calbiochem®
저장 조건
OK to freeze
색상
white to brown
solubility
methanol: 10 mg/mL
water: soluble
배송 상태
ambient
저장 온도
2-8°C
SMILES string
[n]2(cnc(c2N)C(=O)N)[C@@H]1O[C@@H]([C@H]([C@H]1O)O)CO
InChI
1S/C9H14N4O5/c10-7-4(8(11)17)12-2-13(7)9-6(16)5(15)3(1-14)18-9/h2-3,5-6,9,14-16H,1,10H2,(H2,11,17)/t3-,5-,6-,9-/m1/s1
InChI key
RTRQQBHATOEIAF-UUOKFMHZSA-N
일반 설명
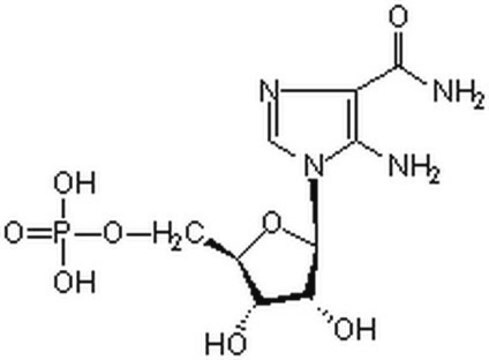
A cell-permeable nucleoside compound that is processed intracellularly to form a phosphorylated metabolite, which activates adenosine monophosphate-activated protein kinase (AMPK) without disrupting the cellular concentrations of ATP, ADP, or AMP. Also acts as a regulator of de novo purine synthesis. Stimulates glucose uptake in both perfused and isolated muscle. AICAr-stimulated glucose transport is not affected by Wortmannin (Cat. No. 681675), a PI-3K inhibitor. Shown to inhibit the synthesis of triacylglycerol (TAG), diacylglycerol (DAG), and phospholipid, probably as a result of AMPK activation and the subsequent inhibition of sn-glycerol-3-phosphate acyltransferase (GPAT) by AMPK. Also reported to inhibit Hsp90 chaperone function. Imparts protection against cell death induced by glucose deprivation, chemical hypoxia, and exposure to glutamate and amyloid β (Aβ) peptide.
A cell-permeable nucleoside compound whose phosphorylated metabolite activates adenosine monophosphate-activated protein kinase (AMPK) and acts as a regulator of de novo purine synthesis. Stimulates glucose uptake in perfused and isolated muscle. Offers protection against cell death induced by glucose deprivation, chemical hypoxia, and exposure to glutamate and Aβ peptide.
생화학적/생리학적 작용
Cell permeable: yes
Primary Target
Adenosine monophosphate-activated protein kinase (AMPK)
Adenosine monophosphate-activated protein kinase (AMPK)
Product does not compete with ATP.
Reversible: no
포장
Packaged under inert gas
경고
Toxicity: Standard Handling (A)
제조 메모
May require heating to 50°C to achieve complete solubilization in methanol.
재구성
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
기타 정보
Meli, M., et al. 2006. J. Med. Chem.in press.
Culmsee, C., et al. 2001. J. Mol. Neurosci.17, 45.
Muoio, D.M., et al. 1999. Biochem. J.338, 783.
Hayashi, T., et al. 1998. Diabetes47, 1369.
Corton, J.M., et al. 1995. Eur. J. Biochem.229, 558.
Culmsee, C., et al. 2001. J. Mol. Neurosci.17, 45.
Muoio, D.M., et al. 1999. Biochem. J.338, 783.
Hayashi, T., et al. 1998. Diabetes47, 1369.
Corton, J.M., et al. 1995. Eur. J. Biochem.229, 558.
법적 정보
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Fansi Jin et al.
Allergy, 74(6), 1145-1156 (2018-12-20)
Nuclear receptor subfamily 4 group A member 1 (NR4A1), an orphan nuclear receptor, has been implicated in several biological events such as metabolism, apoptosis, and inflammation. Recent studies indicate a potential role for NR4A1 in mast cells, yet its role
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.