추천 제품
양식
powder
포장
pkg of 1 × 1 mg (860665P-1mg)
제조업체/상표
Avanti Research™ - A Croda Brand 860665P
지질 유형
sphingolipids
배송 상태
dry ice
저장 온도
−20°C
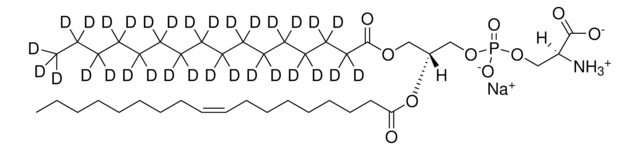
SMILES string
OC[C@@](N)([H])[C@]([H])(O)/C=C/CCCCCCCC/C=C\CCC
InChI
1S/C18H35NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h4-5,14-15,17-18,20-21H,2-3,6-13,16,19H2,1H3/b5-4-,15-14+
InChI key
KWDXKYNWAKMLKK-YQMRQDNGSA-N
일반 설명
4E,14Z-Sphingadiene is a unique sphingoid base containing C18 chain, with trans double bond at C-4 and cis double bond between C14 and C15 with a bent structure. It is found in the plasma, lymph, brain, kidney and lungs of humans and mice. 4E,14Z-Sphingadiene or d-erythro-1,3-dihydroxy-2-amino-4-trans-14-cis-octadecadiene is the second largest long-chain base of human plasma sphingomyelins.
생화학적/생리학적 작용
Sphingadiene, a vital component of sphingolipids facilitates numerous processes such as apoptosis and cell signaling. It inhibits Akt/Wnt signaling and acts as an anticancer agent in colon cancer.
포장
5 mL Amber Glass Screw Cap Vial (860665P-1mg)
법적 정보
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
O Renkonen et al.
Journal of lipid research, 10(6), 687-693 (1969-11-01)
The dienic long-chain base (sphingadienine) of human plasma sphingomyelins has been identified as d-erythro-1,3-dihydroxy-2-amino-4-trans-14-cis-octadecadiene. A similar sphingosine was also detected in plasma sphingomyelins of rat, rabbit, and cat. The key reaction in the structural studies was partial reduction of sphingadienine
Occurrence, structure elucidation, biosynthesis, functions and synthesis of sphingadienes
U Abeytunga T
Mini-Reviews in Organic Chemistry, 12(3), 282-292 (2015)
Keisuke Jojima et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 34(2), 3318-3335 (2020-01-10)
Sphingolipids are multifunctional lipids. Among the sphingolipid-component sphingoid bases, 4,14-sphingadiene (SPD) is unique such that it has a cis double bond with a bent structure. Although SPD was discovered half a century ago, its tissue distribution, biosynthesis, and degradation remain
Sarah T Pruett et al.
Journal of lipid research, 49(8), 1621-1639 (2008-05-24)
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.