추천 제품
양식
powder
포장
pkg of 1 × 1 mg (860464P-1mg)
제조업체/상표
Avanti Research™ - A Croda Brand 860464P
지질 유형
sphingolipids
bioactive lipids
배송 상태
dry ice
저장 온도
−20°C
SMILES string
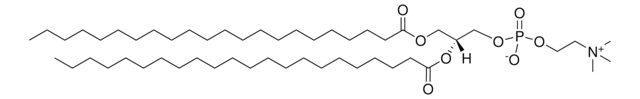
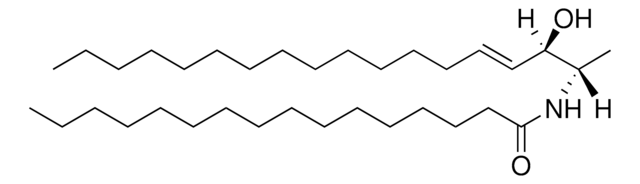
C[C@]([H])(NC(CCCCCCCCCCCCC/C=C\CCCCCCCC)=O)[C@]([H])(O)CCCCCCCCCCCCCCC
InChI key
XBNZFPFCTSJHRI-FIIAPBGQSA-N
일반 설명
1-deoxysphinganine is an atypical sphingoid base, which lacks a 1-hydroxyl group.
애플리케이션
N-C24:1-deoxysphinganine or N-nervonoyl-1-deoxysphinganine (m18:0/24:1) has been used as a standard in the quantitation of atypical sphingoid bases in biological samples by reverse-phase liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-MS/MS).
생화학적/생리학적 작용
1-deoxysphinganine might be toxic to cancer cells. It is a potential biomarker for type 2 diabetes. 1-deoxysphinganine acts as a cytotoxic lipid for insulin producing cells.
포장
5 mL Amber Glass Screw Cap Vial (860464P-1mg)
법적 정보
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
Noemi Jiménez-Rojo et al.
Biophysical journal, 107(12), 2850-2859 (2014-12-18)
Ceramides and dihydroceramides are N-acyl derivatives of sphingosine and sphinganine, respectively, which are the major sphingoid-base backbones of mammals. Recent studies have found that mammals, like certain other organisms, also produce 1-deoxy-(dihydro)ceramides (1-deoxyDHCers) that contain sphingoid bases lacking the 1-hydroxyl-
Junliang Wan et al.
Journal of agricultural and food chemistry, 67(46), 12953-12961 (2019-10-23)
Most common sphingolipids are comprised of "typical" sphingoid bases (sphinganine, sphingosine, and structurally related compounds) and are produced via the condensation of l-serine with a fatty acyl-CoA by serine palmitoyltransferase. Some organisms, including mammals, also produce "atypical" sphingoid bases that
Sarah T Pruett et al.
Journal of lipid research, 49(8), 1621-1639 (2008-05-24)
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

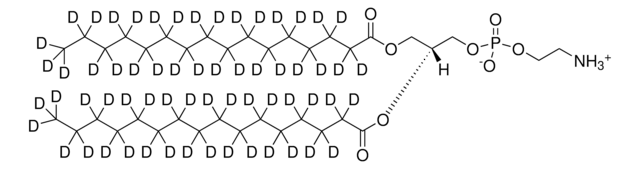
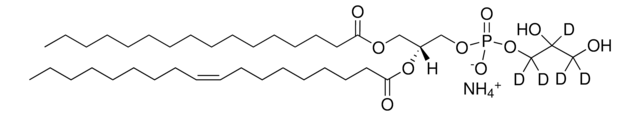
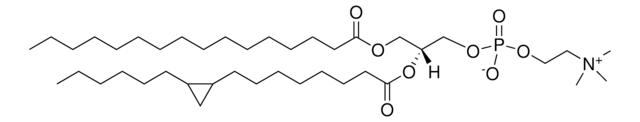
![16:0-d31-18:1 PG 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt), chloroform](/deepweb/assets/sigmaaldrich/product/structures/392/823/d104ed37-ed4a-4f66-8294-a20d5f9085b4/640/d104ed37-ed4a-4f66-8294-a20d5f9085b4.png)