L511188
(S)-2-(2,3-Bis(dicyclohexylamino)cyclopropenimine)-3-phenylpropan-1-ol hydrochloride
AldrichCPR
동의어(들):
(βS)-β-[[2,3-bis(dicyclohexylamino)-2-cyclopropen-1-ylidene]amino]-benzenepropanol hydrochloride (1:1), Dicyclohexyl cyclopropenimine, Lambert Cyclopropenimine Catalyst
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C36H56ClN3O
CAS Number:
Molecular Weight:
582.30
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
추천 제품
SMILES string
Cl.OC[C@H](Cc1ccccc1)\N=C2\C(N(C3CCCCC3)C4CCCCC4)=C2N(C5CCCCC5)C6CCCCC6
InChI
1S/C36H55N3O.ClH/c40-27-29(26-28-16-6-1-7-17-28)37-34-35(38(30-18-8-2-9-19-30)31-20-10-3-11-21-31)36(34)39(32-22-12-4-13-23-32)33-24-14-5-15-25-33;/h1,6-7,16-17,29-33,40H,2-5,8-15,18-27H2;1H/t29-;/m0./s1
InChI key
RTCSAEOYHXMTHG-JMAPEOGHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Learn More at the Professor and Product Portal of Professor Tristan Lambert.
애플리케이션
Chiral cyclopropenimines are highly effective new class of enantioselective Brønsted base catalysts, the so-called “superbases” for enantioselective organocatalysis.
Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products.
Novel Brønsted bases provide potent yet tunable basicity to the acidity of a given substrate, are trivial to prepare, and offer unique opportunities for asymmetric transition state organization. The high basicity should allow them to catalyze a wide range of reactions (i.e. chiral pharmaceutical ingredients) and could lead to easier and faster syntheses of novel chiral compounds for drug discovery and other applications.
2,3-bis(dialkylamino)cyclopropenimines serve as a highly effective platform for chiral Brønsted base catalysis. Chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. Catalysis with chiral cyclopropenimines should be amenable to relatively large-scale applications under mild reaction conditions.

Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products.
Novel Brønsted bases provide potent yet tunable basicity to the acidity of a given substrate, are trivial to prepare, and offer unique opportunities for asymmetric transition state organization. The high basicity should allow them to catalyze a wide range of reactions (i.e. chiral pharmaceutical ingredients) and could lead to easier and faster syntheses of novel chiral compounds for drug discovery and other applications.
2,3-bis(dialkylamino)cyclopropenimines serve as a highly effective platform for chiral Brønsted base catalysis. Chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. Catalysis with chiral cyclopropenimines should be amenable to relatively large-scale applications under mild reaction conditions.

기타 정보
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Jeffrey S Bandar et al.
Journal of the American Chemical Society, 134(12), 5552-5555 (2012-03-16)
Cyclopropenimines are shown to be a highly effective new class of enantioselective Brønsted base catalysts. A chiral 2,3-bis(dialkylamino)cyclopropenimine catalyzes the rapid Michael reaction of a glycine imine substrate with high levels of enantioselectivity. A preparative scale reaction to deliver 25
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.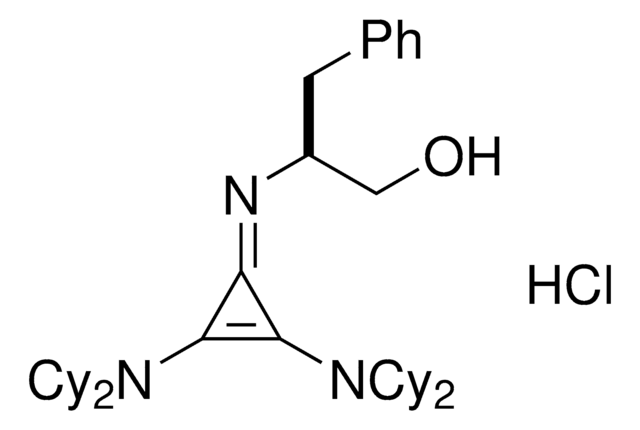

![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)