747130
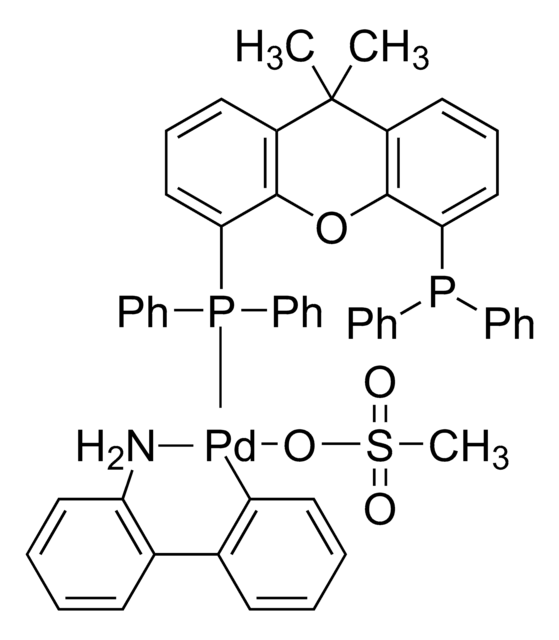
Josiphos SL-J009-1 Pd G3
동의어(들):
Josiphos SL-J009-1-G3-palladacycle, {(R)-1-[(Sp)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine}[2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
추천 제품
형태
solid
Quality Level
특징
generation 3
반응 적합성
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
180-186 °C
작용기
phosphine
SMILES string
NC1=C(C=CC=C1)C2=C([Pd]OS(C)(=O)=O)C=CC=C2.C[C@@H](P(C(C)(C)C)C(C)(C)C)[C]3[C](P(C4CCCCC4)C5CCCCC5)[C][C][C]3.[C]6[C][C][C][C]6.[Fe]
InChI
1S/C27H47P2.C12H10N.C5H5.CH4O3S.Fe.Pd/c1-21(29(26(2,3)4)27(5,6)7)24-19-14-20-25(24)28(22-15-10-8-11-16-22)23-17-12-9-13-18-23;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-2-4-5-3-1;1-5(2,3)4;;/h14,19-23H,8-13,15-18H2,1-7H3;1-6,8-9H,13H2;1-5H;1H3,(H,2,3,4);;/q;;;;;+1/p-1/t21-;;;;;/m1...../s1
InChI key
GJFJVCUFUWXFEB-RPDSBUJNSA-M
일반 설명
애플리케이션
- Total Syntheses of N-Paspaline and N-Emindole PB: Discusses the use of various catalysts including RuPhos and Josiphos ligands for synthesizing complex molecular structures (Radical-Polar Crossover Cyclizations, 2020).
- Unprotected Indazoles Are Resilient to Ring-Opening Isomerization: A Case Study on Catalytic C-S Couplings in the Presence of Strong Base: Utilizes Josiphos SL-J009-1 Pd-G3 for catalytic C-S coupling reactions under strong base conditions (The Journal of Organic Chemistry, 2017).
- Synthesis and characterization of an isopropylBippyPhos precatalyst: Compares various phosphine ligands including XPhos and Josiphos for catalytic activity in organic syntheses (Tetrahedron, 2022).
- Novel quorum sensing inhibitors targeting PqsR: Mentions the experimental use of Josiphos ligands in the synthesis of potential pharmaceutical compounds (2020, University of Saarland).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
문서
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All of the preformed catalysts used in the kit are air and moisture stable complexes in their commercially available form. Once activated by base under the reaction conditions they become sensitive to air. To best enable scale-up success, the use of standard Schlenk technique is recommended.
All contents in the foil bag are weighed, plated, packed, and sealed in a glove box under nitrogen.
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
관련 콘텐츠
Cross-coupling is a common reaction in organic chemistry for the creation of C-C, C-N, and C-O bonds with the aid of a metal catalyst.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/809/974/e027b628-7c2e-4bde-be7e-f9298d0c8b04/640/e027b628-7c2e-4bde-be7e-f9298d0c8b04.png)
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/245/493/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0/640/2ae2dd8a-65cc-4aba-9a1f-1292eb1ad8e0.png)
![(R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/168/768/54a48841-6fe6-437a-81af-8c2e54117ef3/640/54a48841-6fe6-437a-81af-8c2e54117ef3.png)
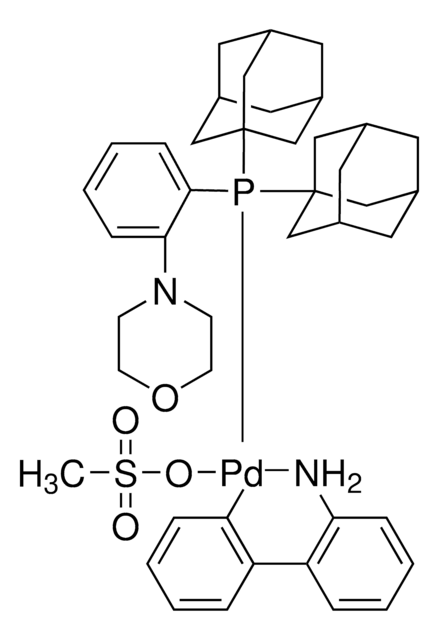
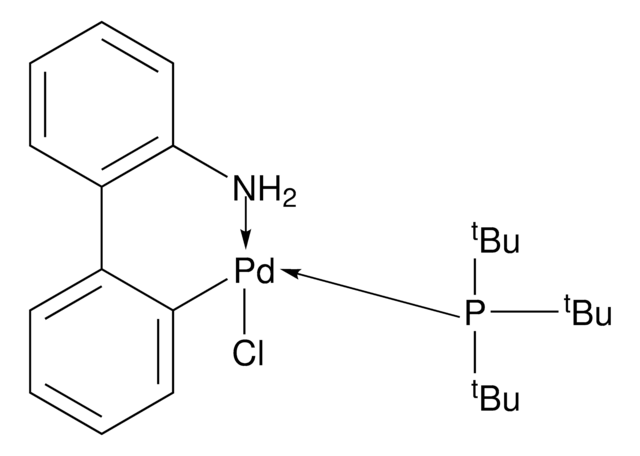
![Mesyl[(tri-t-butylphosphine)-2-(2-aminobiphenyl)]palladium(II)](/deepweb/assets/sigmaaldrich/product/structures/358/298/6539c19e-808c-4cd1-b9e8-19c6928f2384/640/6539c19e-808c-4cd1-b9e8-19c6928f2384.png)
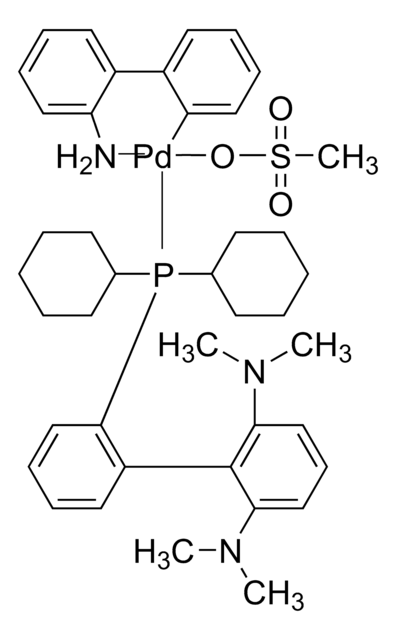
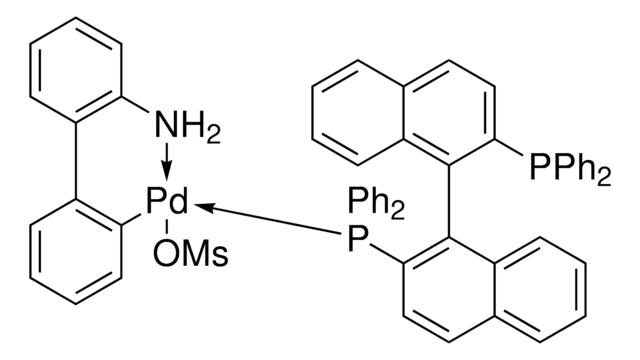
![(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldicyclohexylphosphine ≥97%](/deepweb/assets/sigmaaldrich/product/structures/156/571/6166b550-075e-4d67-b972-a85cb50b2b22/640/6166b550-075e-4d67-b972-a85cb50b2b22.png)
![[(1,3,5,7-Tetramethyl-6-phenyl-2,4,6-trioxa-6-phosphaadamantane)-2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate](/deepweb/assets/sigmaaldrich/product/structures/324/001/3ffb4bd2-9c6b-451c-80ee-a217f03ca932/640/3ffb4bd2-9c6b-451c-80ee-a217f03ca932.png)