추천 제품
Quality Level
분석
98%
bp
153-156 °C/20 mmHg (lit.)
mp
84-86 (lit.)
SMILES string
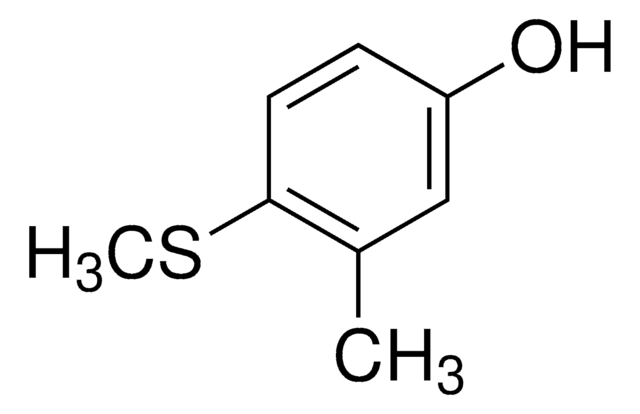
CSc1ccc(O)cc1
InChI
1S/C7H8OS/c1-9-7-4-2-6(8)3-5-7/h2-5,8H,1H3
InChI key
QASBCTGZKABPKX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
4-(Methylmercapto)phenol (4MP) mediates the removal of benzyloxycarbonyl and O-benzyl protecting groups by accepting the benzyl groups during the acidolytic cleavage with trifluoroacetic acid. The presence of hydroxyl group in the para position enhances the rate of hydrodesulfurization (HDS) of 4MP.
애플리케이션
4-(Methylmercapto)phenol [4-(Methylthio)phenol] may be used in the preparation of phosphoramidodithioate intermediates for the synthesis of sulprofos amidate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Lianming Wu et al.
Journal of mass spectrometry : JMS, 44(9), 1389-1394 (2009-08-22)
A novel ion/molecule reaction was observed to occur under electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and atmospheric pressure photo ionization (APPI) conditions, leading to dimerization of ionized 4-(methyl mercapto)-phenol followed by fast H(*) loss. The reaction is particularly
Acceptors in the removal of protecting groups.
Bodanszky M and Bodanszky A.
International Journal of Peptide and Protein Research, 23(3), 287-291 (1984)
Hydrotreating of compounds containing both oxygen and sulfur: effect of para-hydroxyl substituent on the reactions of mercapto and methylmercapto groups.
Viljava TR and Krause AOI.
Applied Catalysis A: General, 145(1), 237-251 (1996)
Resolution and stereoselective action of sulprofos and related S-propyl phosphorothiolates.
Hirashima A, et al.
Journal of Agricultural and Food Chemistry, 32(6), 1302-1307 (1984)
Hua Zhang et al.
Molecules (Basel, Switzerland), 15(1), 83-92 (2010-01-30)
A highly efficient transition-metal-free catalytic system Br2/NaNO2/H2O has been developed for a robust and economic acid-free aerobic oxidation of sulfides. It is noteworthy that the sulfide function reacts under mild conditions without over-oxidation to sulfone. The role of NaNO2as an
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.