모든 사진(1)
About This Item
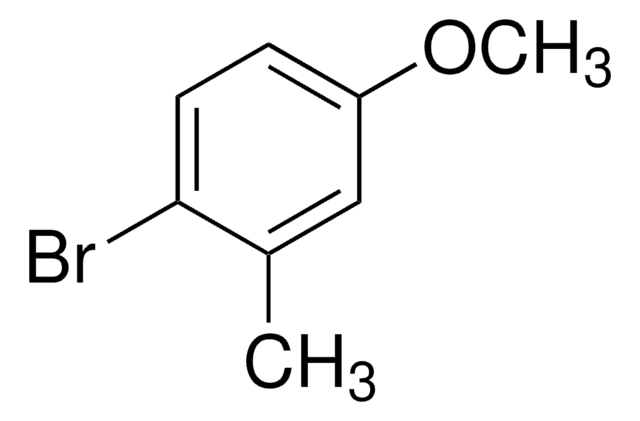
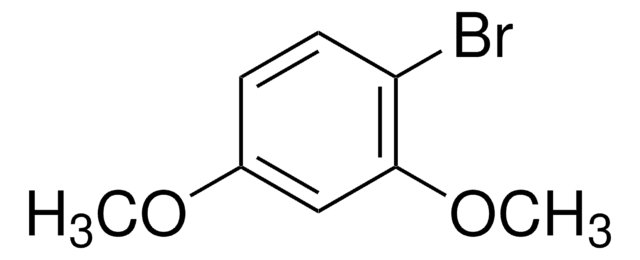
Linear Formula:
BrC6H3(CH3)OCH3
CAS Number:
Molecular Weight:
201.06
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
양식
solid
mp
66-69 °C (lit.)
작용기
bromo
SMILES string
COc1ccc(Br)cc1C
InChI
1S/C8H9BrO/c1-6-5-7(9)3-4-8(6)10-2/h3-5H,1-2H3
InChI key
UDLRGQOHGYWLCS-UHFFFAOYSA-N
일반 설명
4-Bromo-2-methylanisole can be prepared by the bromination of o-methylanisole (2-methylanisole). It can also be prepared via reaction between 1-butyl-3-methylimidazolium tribromide [(Bmim)Br3] and an activated aromatic compound. It participates as a substrate for the a-arylation of sulphonamide in a study.
애플리케이션
4-Bromo-2-methylanisole may be used in the synthesis of 4-isopropyl-2-methylphenol and 4-methoxy-3-methylphenol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
(Bmim) Br3 as a New Reagent for Regioselective Monobromination of Phenols and Several Activated Aromatics under Solvent-free Conditions.
Le ZG, et al.
Chin. J. Chem., 23(11), 1537-1540 (2005)
James R Vyvyan et al.
The Journal of organic chemistry, 69(7), 2461-2468 (2004-03-31)
Aromatic bisabolene derivatives were prepared by two methods involving cross-coupling of organozinc reagents. The first synthesis of (+/-)-glandulone A (10), as well as syntheses of (+/-)-curcuhydroquinone (8) and (+/-)-curcuquinone (9), were accomplished via coupling of a secondary alkyl zinc reagent
Total synthesis of (?)-heliannuol D, an allelochemical from Helianthus annuus.
Vyvyan JR and Looper RE.
Tetrahedron Letters, 41(8), 1151-1154 (2000)
The isopropyl cresols.
Carpenter MS and Easter WM.
The Journal of Organic Chemistry, 20(4), 401-411 (1955)
George Majetich et al.
The Journal of organic chemistry, 62(13), 4321-4326 (1997-06-27)
It has been shown that bromodimethylsulfonium bromide, generated in situ by treating dimethyl sulfoxide with aqueous hydrobromic acid, is a milder and more selective reagent for electrophilic aromatic bromination than elemental bromine.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.