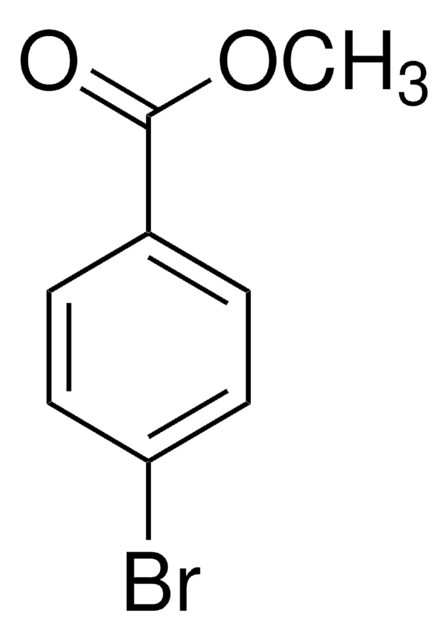
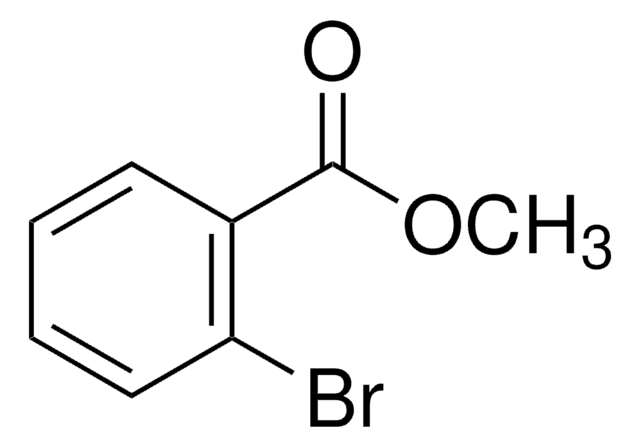
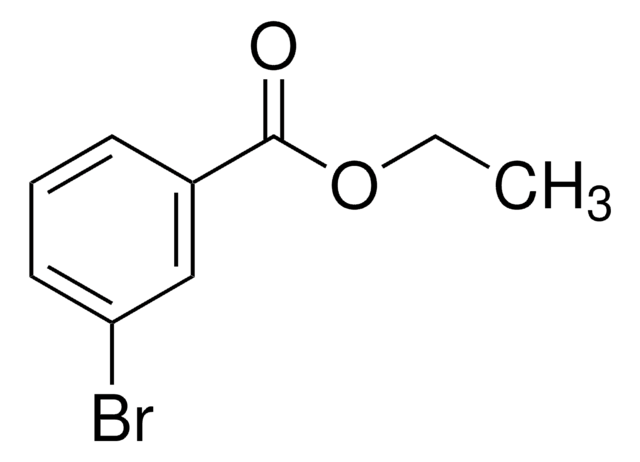
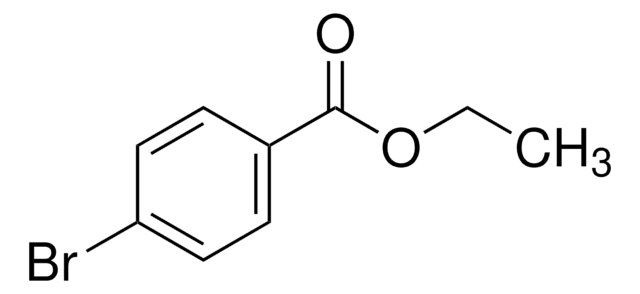
추천 제품
Quality Level
분석
98%
bp
127-128 °C/15 mmHg (lit.)
mp
31-33 °C (lit.)
작용기
bromo
ester
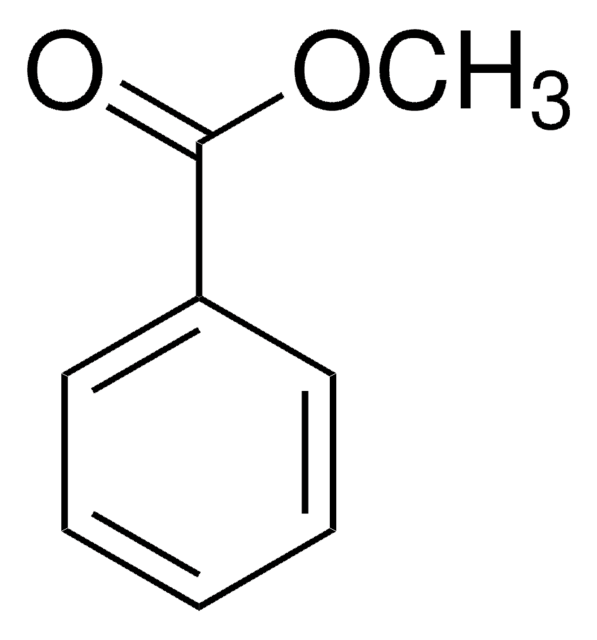
SMILES string
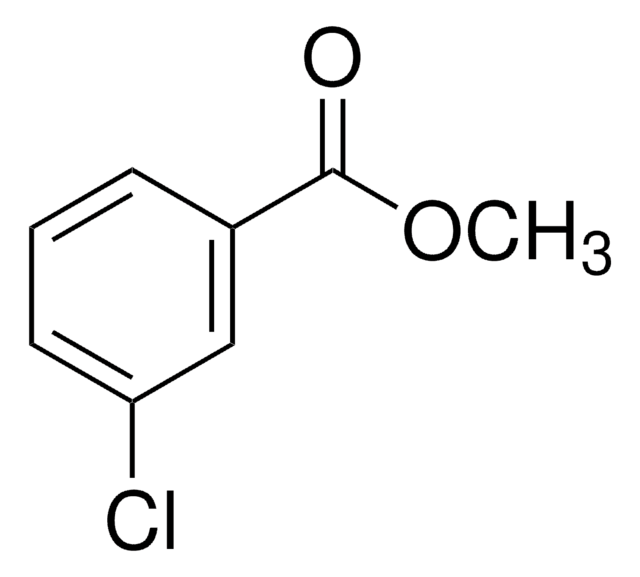
COC(=O)c1cccc(Br)c1
InChI
1S/C8H7BrO2/c1-11-8(10)6-3-2-4-7(9)5-6/h2-5H,1H3
InChI key
KMFJVYMFCAIRAN-UHFFFAOYSA-N
일반 설명
Methyl 3-bromobenzoate is an aryl bromide. It undergoes stereoconvergent cross-coupling with potassium trifluoro(1-phenylethyl)borate to form 1,1-diarylethane derivative. The Negishi cross-coupling reaction between methyl 3-bromobenzoate and diarylzinc reagents in the presence of a palladium catalyst has been reported. Methyl-3-bromobenzoate can be converted into the corresponding benzonitrile using dichloro[bis{1-(dicyclohexylphosphanyl)piperidine}]palladium as a C-C cross-coupling catalyst and K4[Fe(CN)6] as a cyanating agent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Negishi cross-coupling reaction catalyzed by an aliphatic, phosphine based pincer complex of palladium. biaryl formation via cationic pincer-type PdIV intermediates.
Gerber R, et al.
Dalton Transactions, 40(35), 8996-9003 (2011)
Roman Gerber et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(10), 2978-2986 (2012-02-03)
Dichloro[bis{1-(dicyclohexylphosphanyl)piperidine}]palladium [(P{(NC(5)H(10))(C(6)H(11))(2)})(2)PdCl(2)] (1) is a highly active and generally applicable C-C cross-coupling catalyst. Apart from its high catalytic activity in Suzuki, Heck, and Negishi reactions, compound 1 also efficiently converted various electronically activated, nonactivated, and deactivated aryl bromides, which may
John C Tellis et al.
Science (New York, N.Y.), 345(6195), 433-436 (2014-06-07)
The routine application of C(sp3)-hybridized nucleophiles in cross-coupling reactions remains an unsolved challenge in organic chemistry. The sluggish transmetalation rates observed for the preferred organoboron reagents in such transformations are a consequence of the two-electron mechanism underlying the standard catalytic
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.