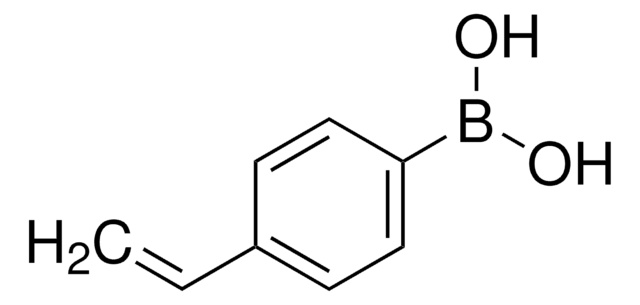
473790
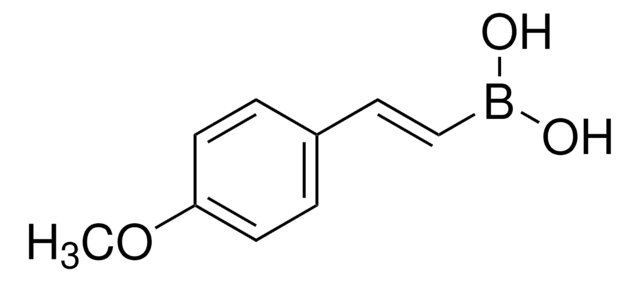
trans-2-Phenylvinylboronic acid
97%
동의어(들):
(E)-2-phenyl-Etheneboronic acid, (E)-Phenylethenylboronic acid, (E)-Styreneboronic acid, (E)-Styrylboronic acid, trans-(2-Phenylethenyl)boronic acid, trans-Phenylvinyl boronic acid
로그인조직 및 계약 가격 보기
모든 사진(2)
About This Item
Linear Formula:
C6H5CH=CHB(OH)2
CAS Number:
Molecular Weight:
147.97
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
mp
146-156 °C (lit.)
작용기
phenyl
SMILES string
OB(O)\C=C\c1ccccc1
InChI
1S/C8H9BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7,10-11H/b7-6+
InChI key
VKIJXFIYBAYHOE-VOTSOKGWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Reagent used for
Reagent used in Preparation of
- Palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions
- Rhodium (Rh)-catalyzed intramolecular amination of aryl azides
- Diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction
- Copper (Cu)-mediated cyanation
- Rhodium (Rh)-catalyzed asymmetric addition
- Diastereoselective synthesis via iridium (Ir)-catalyzed addition
- Palladium (Pd)-catalyzed cascade cyclization
Reagent used in Preparation of
- Optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reaction
- Amino alcohol dienes via Petasis 3-component reaction using Ru-catalyzed ring-closing metathesis and isomerization
기타 정보
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Tomohiro Iwai et al.
Journal of the American Chemical Society, 134(2), 1268-1274 (2011-12-14)
Iridium complexes show high catalytic activity in intermolecular additions of acid chlorides to terminal alkynes to afford valuable (Z)-β-chloro-α,β-unsaturated ketones. Ligands in the catalytic system play a crucial role in this reaction. An N-heterocyclic carbene (NHC) is an efficient ligand
Xiangqing Feng et al.
Organic letters, 14(2), 624-627 (2012-01-12)
This paper describes a Rh(I)-catalyzed highly efficient and enantioselective 1,2-addition of arylboronic acids to α-diketones with the use of a simple sulfur-alkene hybrid ligand. With as low as a 0.1 mol % catalyst loading, a variety of optically active α-hydroxyketones
Ligand Effects on the Stereochemical Outcome of Suzuki-Miyaura Couplings
Lu, G-P.; et al.
The Journal of Organic Chemistry, 77, 370-3703 (2012)
Erhad Ascic et al.
ACS combinatorial science, 14(4), 253-257 (2012-02-24)
A "build/couple/pair" pathway for the systematic synthesis of structurally diverse small molecules is presented. The Petasis 3-component reaction was used to synthesize anti-amino alcohols displaying pairwise reactive combinations of alkene moieties. Upon treatment with a ruthenium alkylidene-catalyst, these dienes selectively
Diastereoselective synthesis of tetrahydroquinolines via a palladium-catalyzed Heck-Suzuki cascade reaction
Wilson, J. E.
Tetrahedron Letters, 53, 2308-2311 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)