모든 사진(1)
About This Item
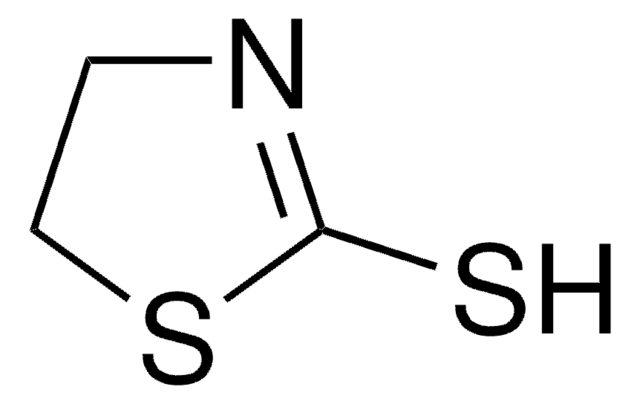
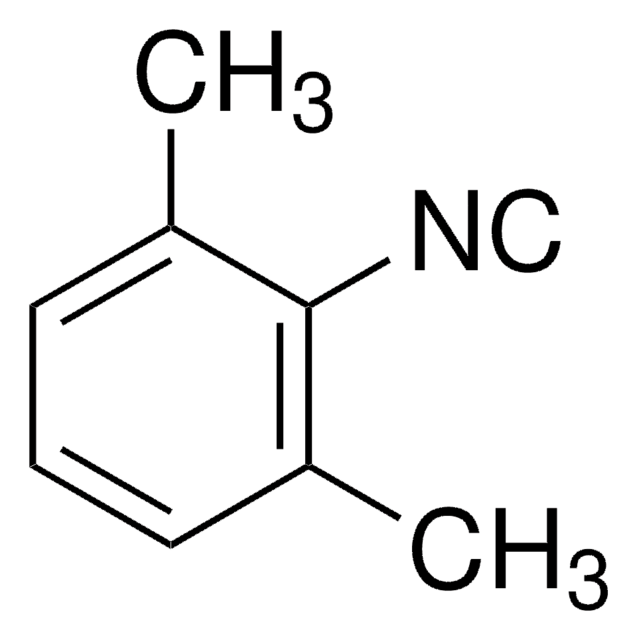
실험식(Hill 표기법):
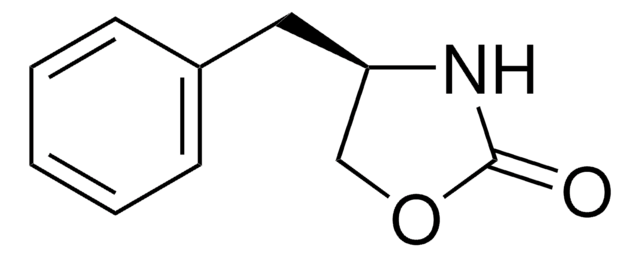
C10H11NS2
CAS Number:
Molecular Weight:
209.33
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
≥97.0%
광학 활성
[α]20/D +122±5°, c = 1% in chloroform
광학 순도
enantiomeric ratio: ≥99:1 (LC)
작용기
phenyl
thioether
SMILES string
S=C1N[C@@H](CS1)Cc2ccccc2
InChI
1S/C10H11NS2/c12-10-11-9(7-13-10)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,11,12)/t9-/m1/s1
InChI key
SLDUGQISGRPGAW-SECBINFHSA-N
애플리케이션
A highly selective and efficient chiral auxiliary which can be directly reduced to its corresponding aldehyde and the chiral auxiliary by reductive cleavage with diisobutylaluminum hydride.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Velazquez. F.; Olivo, H. F.
Current Organic Chemistry, 6, 303-303 (2002)
M T Crimmins et al.
Organic letters, 2(6), 775-777 (2001-02-07)
[formula: see text] Asymmetric aldol additions using chlorotitanium enolates of thiazolidinethione propionates proceed with high diastereoselectivity for the "Evans" or "non-Evans" syn product depending on the nature and amount of the base used. With (-)-sparteine as the base, selectivities of
문서
The asymmetric aldol reaction mediated by chiral auxiliaries is considered to be one of the most important methods for asymmetric C-C bond formation.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.