모든 사진(2)
About This Item
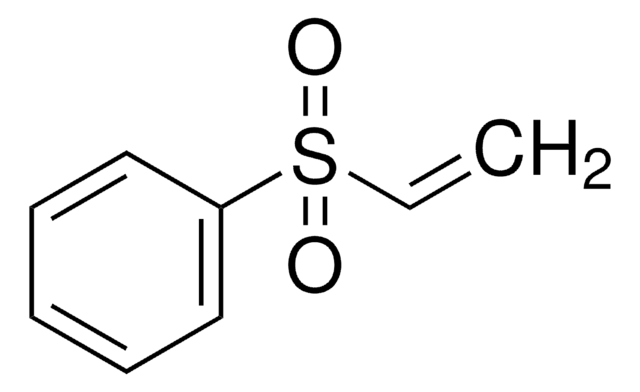
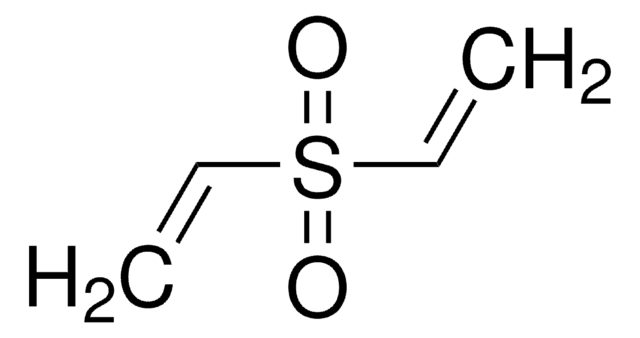
Linear Formula:
C6H5SOCH=CH2
CAS Number:
Molecular Weight:
152.21
Beilstein:
2039218
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
95%
양식
liquid
refractive index
n20/D 1.585 (lit.)
bp
93-95 °C/0.2 mmHg (lit.)
density
1.139 g/mL at 25 °C (lit.)
작용기
sulfoxide
저장 온도
2-8°C
SMILES string
C=CS(=O)c1ccccc1
InChI
1S/C8H8OS/c1-2-10(9)8-6-4-3-5-7-8/h2-7H,1H2
InChI key
MZMJHXFYLRTLQX-UHFFFAOYSA-N
유전자 정보
human ... LOC129293(129293)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Phenyl vinyl sulfoxide reacts with lithium enolates of ketones at -78°C in THF to yield bicyclo[n.2.0]alkan-1-ols. It also reacts with in situ generated (dialkylamino)magnesium reagent to yield symmetrical β-(dialkylamino)dithioacetals. It participates as an acetylene equivalent in Diels-Alder reactions.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
230.0 °F - closed cup
Flash Point (°C)
110 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
X Ji et al.
Acta crystallographica. Section B, Structural science, 45 ( Pt 1), 93-99 (1989-02-01)
Three diastereomers of bicyclo[2.2.1]hept-5-en-2-yl phenyl sulfoxide were prepared by Diels-Alder [4 + 2] cycloadditions between phenyl vinyl sulfoxide and cyclopentadiene. The isomers were separated by column chromatography on silica gel and repeated recrystallizations gave the pure racemates of three of
Masataka Kawakita et al.
The Journal of organic chemistry, 62(23), 8015-8017 (2001-10-24)
Vinyl sulfoxides (PhSOCR(1)=CHR(2): R(1) = H, Me, or Ph; R(2) = H or Me) were treated with (dialkylamino)magnesium reagents, generated in situ from the reaction of EtMgBr with secondary amines (R(3)R(4)NH: R(3) = Et, i-Pr, or Bn; R(4) = Me
Use of phenyl vinyl sulfoxide as an acetylene equivalent in Diels-Alder cycloadditions.
Paquette LA, et al.
Journal of the American Chemical Society, 100(5), 1597-1599 (1978)
Wendy A Loughlin et al.
Organic & biomolecular chemistry, 1(8), 1347-1353 (2003-08-22)
The enolates generated from cyclopentanone, cycloheptanone or cyclooctanone and LDA at -78 degrees C in THF react with (+/-)-phenyl vinyl sulfoxide under controlled conditions of temperature, reaction time, and concentration. Upon oxidation with MCPBA of the product mixtures, the novel
문서
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.