추천 제품
Quality Level
분석
97%
mp
75-77 °C (lit.)
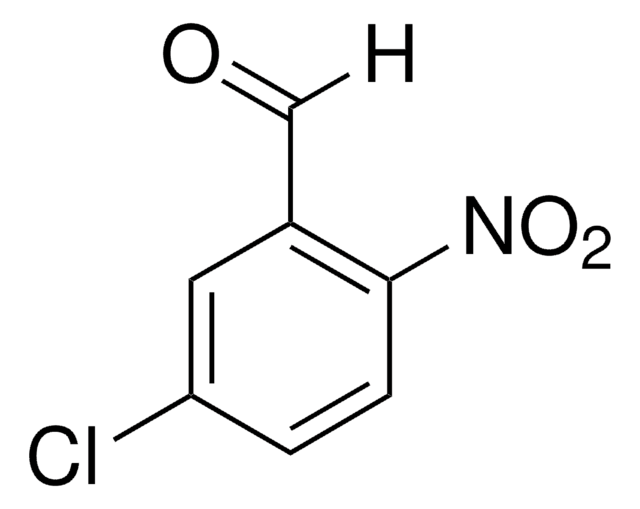
작용기
aldehyde
chloro
nitro
SMILES string
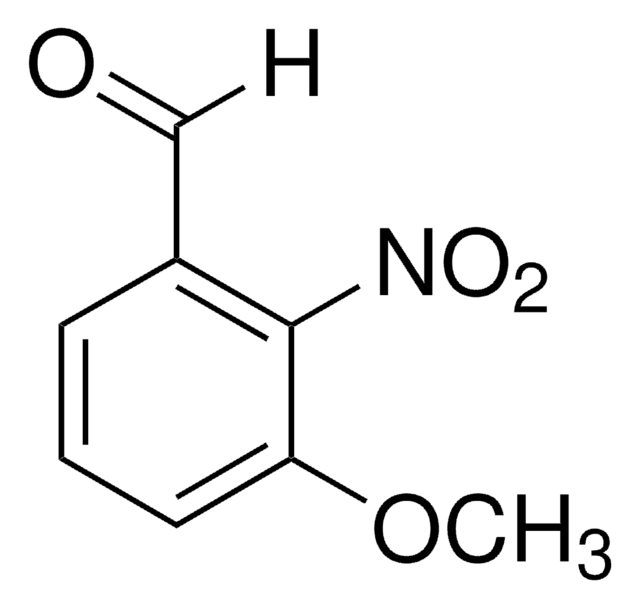
[H]C(=O)c1cc(ccc1Cl)[N+]([O-])=O
InChI
1S/C7H4ClNO3/c8-7-2-1-6(9(11)12)3-5(7)4-10/h1-4H
InChI key
VFVHWCKUHAEDMY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2-Chloro-5-nitrobenzaldehyde undergoes condensation reaction with 2-methyl-1-propenylbenzimidazole to yield (E,Z)-2-(2-chloro-5-nitrostyryl)-1-(1-propenyl)benzimidazole. It reacts with 5-aminopyrazoles to yield symmetrical bispyrazolo[3,4-]pyridines.
애플리케이션
2-Chloro-5-nitrobenzaldehyde was used in the synthesis of 5-nitro-2-(1H-pyrrol-1-yl)benzaldehyde.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Reactions of 5-amino-1, 2-azoles with aromatic and heterocyclic o-chloroaldehydes:[1+ 1] versus [2+ 1] cyclocondensation.
Abramov MA, et al.
Tetrahedron, 57(44), 9123-9129 (2001)
D E Bacelo et al.
Acta crystallographica. Section C, Crystal structure communications, 53 ( Pt 7), 907-909 (1997-07-15)
The title compound, C18H14ClN3O2, was synthesized by the condensation of 2-chloro-5-nitrobenzaldehyde with 2-methyl-1-propenylbenzimidazole, and the molecule comprises a 2-chloro-5-nitrobenzene and a 1-(Z)-propenylbenzimidazole. The two aromatic moieties are conjugated through the vinyl group. The dihedral angle between the two rings is
Morita-Baylis-Hillman route to 4H-pyrrolo [1, 2-a][1] benzazepine derivatives.
Park SP, et al.
Tetrahedron, 65(24), 4703-4708 (2009)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.