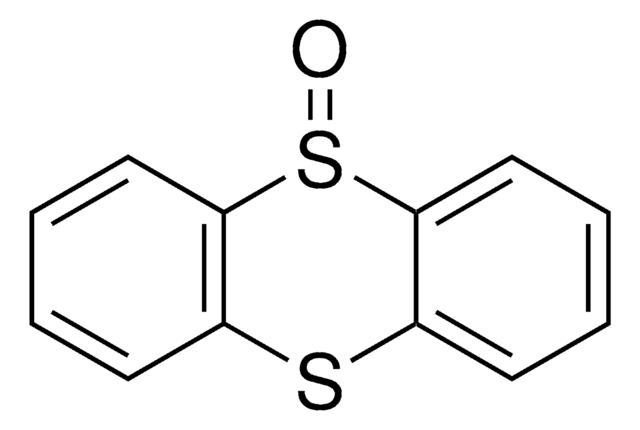
추천 제품
분석
97%
형태
solid
bp
364-366 °C (lit.)
mp
151-155 °C (lit.)
solubility
DMF: soluble
H2O: insoluble
SMILES string
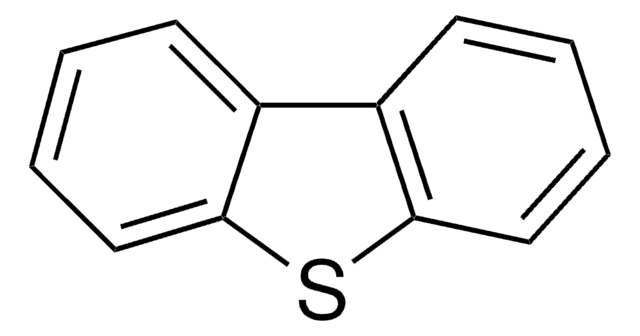
S1c2ccccc2Sc3ccccc13
InChI
1S/C12H8S2/c1-2-6-10-9(5-1)13-11-7-3-4-8-12(11)14-10/h1-8H
InChI key
GVIJJXMXTUZIOD-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Thianthrene undergoes liquid phase tert-butylation in the presence of large pore zeolites and mesoporous aluminosilicates catalyst to yield tert-butyl derivatives. Thianthrene on oxidation in the presence of hydrogen peroxide and ligninase as catalyst from Phanerochaete chrysosporium yields thianthrene monosulfoxide.
애플리케이션
Thianthrene has been used to study aqueous solubilities of several solid nitrogen-, sulfur- and oxygen-containing heterocyclic derivatives of anthracene, phenanthrene and fluorene. It has been used in determination of partition coefficients of several sulfur-containing aromatics in 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and supercritical carbon dioxide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Pavel Karásek et al.
Journal of chromatography. A, 1140(1-2), 195-204 (2006-12-05)
We report the aqueous solubilities of phenanthrene and several solid three-ring aromatic heterocycles (phenanthridine, acridine, phenazine, thianthrene, phenothiazine, phenoxathiin, phenoxazine, carbazole, dibenzofuran, dibenzothiophene, and 4,6-dimethyldibenzothiophene) at temperatures ranging from 313K to the solute melting point and at a pressure of
Acid zeolites as catalysts in organic reactions.< i> tert</i>-Butylation of anthracene, naphthalene and thianthrene.
Armengol E, et al.
Applied Catalysis A: General, 149(2), 411-423 (1997)
Distribution of sulfur-containing aromatics between [hmim][Tf2N] and supercritical CO2: a case study for deep desulfurization of oil refinery streams by extraction with ionic liquids.
Planeta J, et al.
Green Chemistry, 8(1), 70-77 (2006)
R P Schreiner et al.
Applied and environmental microbiology, 54(7), 1858-1860 (1988-07-01)
The oxidation of heterocyclic sulfur compounds reported to be part of the macrostructure of coal and petroleum was investigated. The oxidation of thianthrene solubilized in 10% dimethylformamide to thianthrene monosulfoxide in the presence of hydrogen peroxide was catalyzed by the
Saowapak Kaenkaew et al.
Journal of molecular modeling, 16(2), 243-253 (2009-07-10)
The structures of thiacalix[2]thianthrene, p-tert-butylthiacalix[2]thianthrene and their complexes with Zn(2+), Cd(2+) and Hg(2+) were obtained using B3LYP/LanL2DZ and HF/LanL2DZ calculations. The structures of the most stable conformers of thiacalix[2]thianthrene and p-tert-butylthiacalix[2]thianthrene optimized at either the B3LYP/LanL2DZ or HF/LanL2DZ level are
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.