R7269
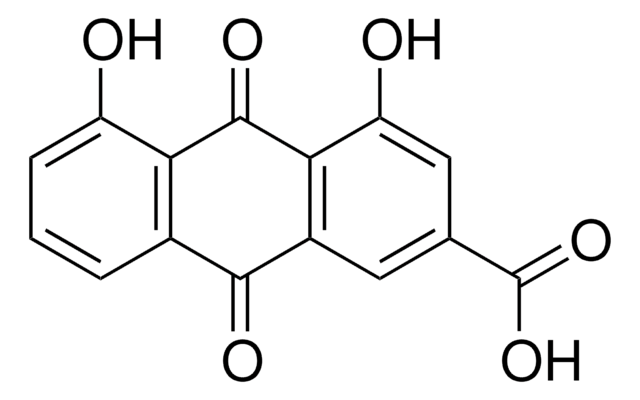
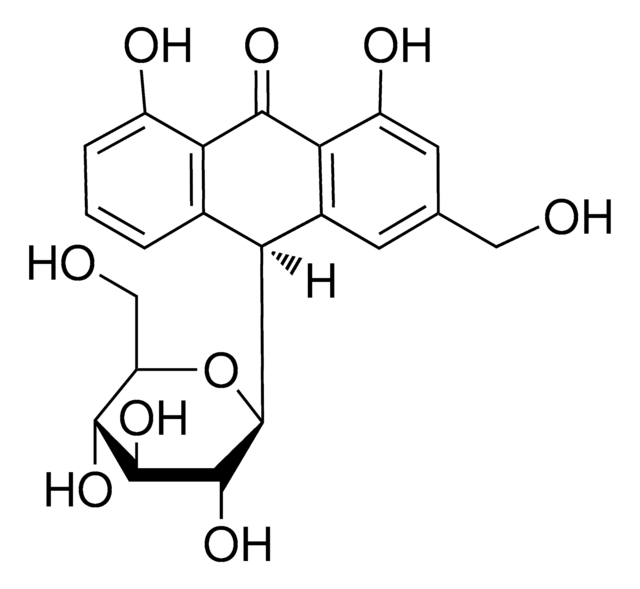
Rhein
≥98% (HPLC), powder, cytotoxic agent
Synonym(s):
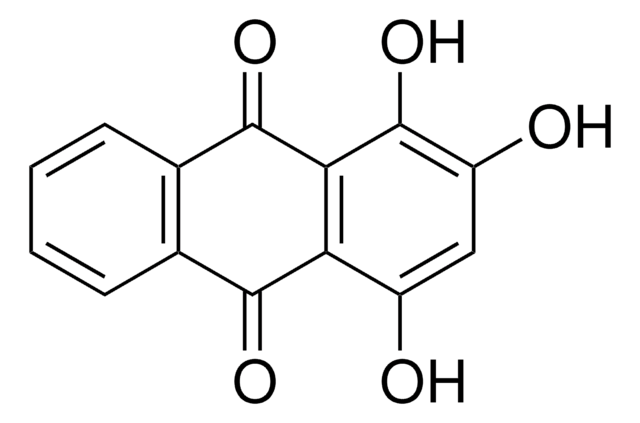
4,5-Dihydroxyanthraquinone-2-carboxylic acid, 9,10-Dihydro-4,5-dihydroxy-9,10-dioxo-2-anthracenecarboxylic acid, Cassic acid, Chrysazin 3-carboxylic acid, Monorhein, Rheic acid, Rhubarb yellow
About This Item
Recommended Products
Product Name
Rhein,
Quality Level
mp
≥300 °C (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)c1cc(O)c2C(=O)c3c(O)cccc3C(=O)c2c1
InChI
1S/C15H8O6/c16-9-3-1-2-7-11(9)14(19)12-8(13(7)18)4-6(15(20)21)5-10(12)17/h1-5,16-17H,(H,20,21)
InChI key
FCDLCPWAQCPTKC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as a potassium simplex optimization medium with amino acids (KSOMaa) to induce degeneration in embryos to study its effect on the intracellular and extracellular micro-RNA (miRNA) signature
- as a fat mass and obesity-associated protein (FTO) inhibitor to study its role in motile ciliogenesis in an m6A-dependent manner in Xenopus embryos
- as an FTO inhibitor to infer its antiviral action in relation with the SARS-associated coronavirus 2 (SARS-CoV-2) infection
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service