P8511
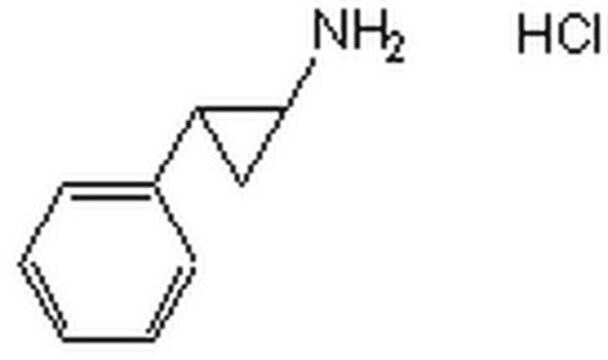
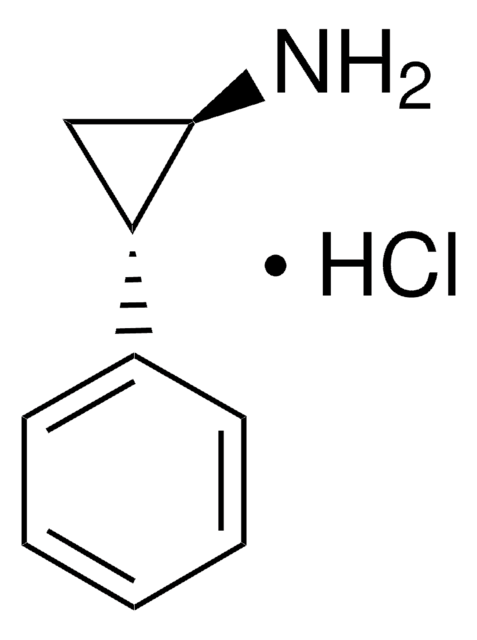
trans-2-Phenylcyclopropylamine hydrochloride
Synonym(s):
Tranylcypromine
About This Item
Recommended Products
biological source
synthetic (organic)
Assay
≥97% (TLC)
form
powder
mp
162-169 °C (lit.)
solubility
ethanol: 50 mg/mL, clear to slightly hazy
storage temp.
2-8°C
SMILES string
Cl.N[C@@H]1C[C@H]1c2ccccc2
InChI
1S/C9H11N.ClH/c10-9-6-8(9)7-4-2-1-3-5-7;/h1-5,8-9H,6,10H2;1H/t8-,9+;/m0./s1
InChI key
ZPEFMSTTZXJOTM-OULXEKPRSA-N
Gene Information
human ... MAOA(4128) , MAOB(4129)
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service