P1431
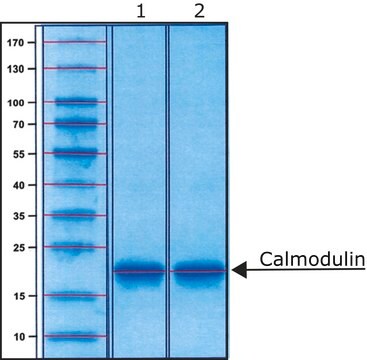
Calmodulin from bovine testes
BioUltra, ≥98% (SDS-PAGE), lyophilized powder, essentially salt free
Synonym(s):
CaM, Phosphodiesterase 3′:5′-cyclic nucleotide activator
About This Item
Recommended Products
biological source
bovine testis
Quality Level
product line
BioUltra
Assay
≥98% (SDS-PAGE)
form
lyophilized powder
mol wt
16.79 kDa
storage condition
(Keep container tightly closed in a dry and well-ventilated place)
technique(s)
ligand binding assay: suitable
impurities
salt, essentially free
UniProt accession no.
application(s)
cell analysis
storage temp.
−20°C
Gene Information
cow ... CALM3(520277)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Calmodulin (CaM) is a Ca2+-sensor protein containing four EF-hand motifs that bind to four Ca2+ ions. It is found ubiquitously in all eukaryotes.
Application
- as a component of the reaction mixture in PhosphoSens assay to measure Ca2+/calmodulin-dependent protein kinase II α (CaMKIIα) substrate phosphorylation
- to generate standard curve for the determination of in situ calmodulin concentration in tissues
- as a ligand in radio-ligand binding for studying calmodulin affinity
Biochem/physiol Actions
antibody
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service