K4769
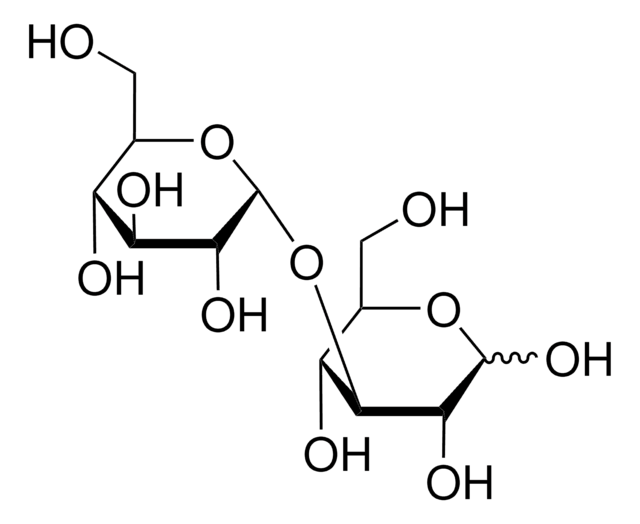
Kojibiose
≥98% (HPLC)
Synonym(s):
α-D-Glc-(1→2)-D-Glc, 2-O-α-D-Glucopyranosyl-D-glucose
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H22O11
CAS Number:
Molecular Weight:
342.30
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
technique(s)
HPLC: suitable
color
white to off-white
solubility
water: 5 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
OCC(O)C(O)C(O)C(OC1OC(CO)C(O)C(O)C1O)C=O
InChI
1S/C12H22O11/c13-1-4(16)7(17)8(18)5(2-14)22-12-11(21)10(20)9(19)6(3-15)23-12/h2,4-13,15-21H,1,3H2
InChI key
PZDOWFGHCNHPQD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Kojibiose, a disaccharide product of glucose caramelization and an inhibitor of plant glucosidase I, may be used to help identify and characterize glucosidase I enzymes involved in terminal deglycosylation of high-mannose oligosaccharides. Kojibiose may be used as a substrate to study the biological species, enzymes and catabolic processes that catabolize it as an energy source. Kojibiose may be used to identify, differentiate and characterize kojibiose phosphorylase(s) (KP).
Biochem/physiol Actions
Kojibiose is an inhibitor of plant glucosidase I. It inhibits the removal of terminal glucose from the high-mannose oligosaccharide (Glc)3(Man)9(GlcNAc)2, either from the free oligosaccharide or from the oligosaccharide attached to a protein via N-linkage.
Other Notes
To gain a comprehensive understanding of our extensive range of Disaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jong-Hyun Jung et al.
Journal of bacteriology, 196(5), 1122-1131 (2014-01-07)
A unique gene cluster responsible for kojibiose utilization was identified in the genome of Pyrococcus sp. strain ST04. The proteins it encodes hydrolyze kojibiose, a disaccharide product of glucose caramelization, and form glucose-6-phosphate (G6P) in two steps. Heterologous expression of
Satoshi Okada et al.
The FEBS journal, 281(3), 778-786 (2013-11-22)
Glycoside hydrolase (GH) family 65 contains phosphorylases acting on maltose (Glc-α1,4-Glc), kojibiose (Glc-α1,2-Glc), trehalose (Glc-α1,α1,-Glc), and nigerose (Glc-α1,3-Glc). These phosphorylases can efficiently catalyze the reverse reactions with high specificities, and thus can be applied to the practical synthesis of α-glucosyl
M K Dowd et al.
Carbohydrate research, 230(2), 223-244 (1992-06-16)
Energy surfaces were computed for relative orientations of the relaxed pyranosyl rings of the two anomeric forms of kojibiose, nigerose, and maltose, the (1----2)-alpha, (1----3)-alpha, and (1----4)-alpha-linked D-glucosyl disaccharides, respectively. Twenty-four combinations of starting conformations of the rotatable side-groups were
Wouter F J Hogendorf et al.
Bioorganic & medicinal chemistry, 18(11), 3668-3678 (2010-04-23)
In this paper the synthesis of an Enterococcus Faecalis teichoic acid (TA) hexamer is presented. The key kojibiosyl-glycerol phosphoramidite building block was obtained by condensation of thioglucose donors, provided with various protecting groups at the C2 hydroxyl function with an
A R Santa Cruz et al.
Experientia, 41(7), 928-929 (1985-07-15)
We have prepared dolichylpyrophosphoryl-[14C]-oligosaccharide (Dol-PP-oligosaccharide) from calf thyroid. Microsomal fractions from human breast tissues catalyzed the transfer of labeled oligosaccharide to endogenous acceptor proteins. Malignant tumors showed higher activity of the oligosaccharide transferring enzyme than normal tissue. With kojibiose (Kj)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service