E8284
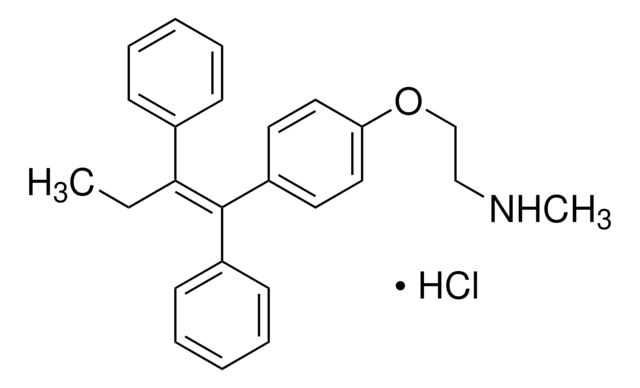
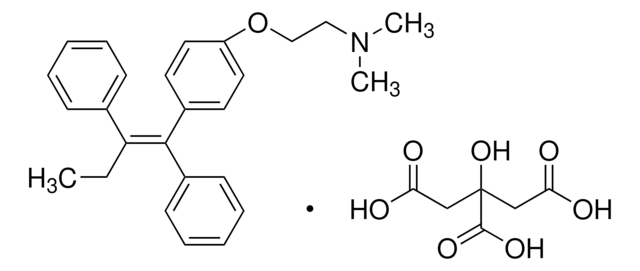
(E/Z)-Endoxifen Hydrochloride Hydrate
≥98% (HPLC)
Synonym(s):
(E/Z)-Endoxifen hydrochloride hydrate, (E/Z)-4-Hydroxy-N-desmethyltamoxifen hydrochloride hydrate, (E/Z)-4-[1-[4-[2-(Methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]-phenol hydrochloride hydrate (1:1 E/Z mixture); (E/Z)-4OHNDtam hydrochloride hydrate
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
solid
storage condition
desiccated
color
white to beige
solubility
DMSO: >10 mg/mL
storage temp.
2-8°C
SMILES string
O.Cl.CC\C(c1ccccc1)=C(/c2ccc(O)cc2)c3ccc(OCCNC)cc3
InChI
1S/C25H27NO2.ClH.H2O/c1-3-24(19-7-5-4-6-8-19)25(20-9-13-22(27)14-10-20)21-11-15-23(16-12-21)28-18-17-26-2;;/h4-16,26-27H,3,17-18H2,1-2H3;1H;1H2/b25-24-;;
InChI key
BISAMGUAXFFKDX-NDIQBZANSA-N
Application
as a selective estrogen receptor modulator (SERM) to study the role of miR-27a expression
to impregnate a biocompatible polymer in the subcutaneous space adjacent to the TA muscle of PDGFRαCreER;R26NG mice
to study its in vivo performance and properties for CreERT2 (mutant estrogen receptor) control
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service