C1172
ML-9
≥99% (TLC), powder
Synonym(s):
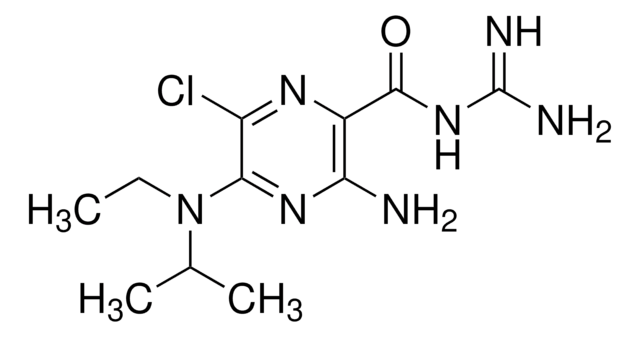
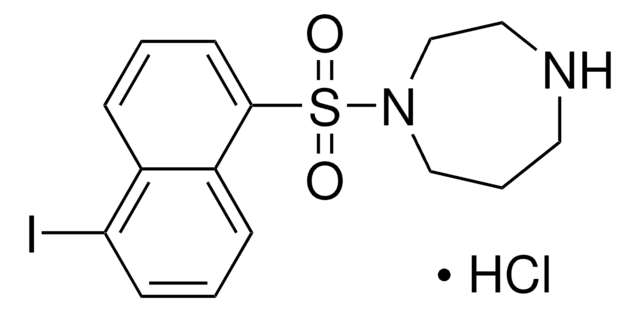
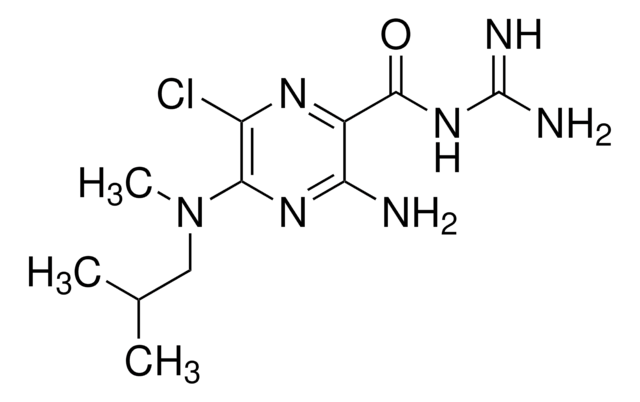
1-(5-Chloronaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine hydrochloride
About This Item
Recommended Products
biological source
synthetic (organic)
Assay
≥99% (TLC)
form
powder
color
white
solubility
ethanol: 10 mg/mL
storage temp.
2-8°C
SMILES string
Cl.Clc1cccc2c(cccc12)S(=O)(=O)N3CCCNCC3
InChI
1S/C15H17ClN2O2S.ClH/c16-14-6-1-5-13-12(14)4-2-7-15(13)21(19,20)18-10-3-8-17-9-11-18;/h1-2,4-7,17H,3,8-11H2;1H
InChI key
ZNRYCIVTNLZOGI-UHFFFAOYSA-N
Gene Information
human ... SLC2A1(6513) , SLC2A4(6517)
Application
Biochem/physiol Actions
Features and Benefits
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Glucose metabolism is regulated by the opposing actions of insulin and glucagon. Insulin is released from pancreatic ß cells in response to high blood glucose levels and regulates glucose metabolism through its actions on muscle, liver, and adipose tissue.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service