A6805
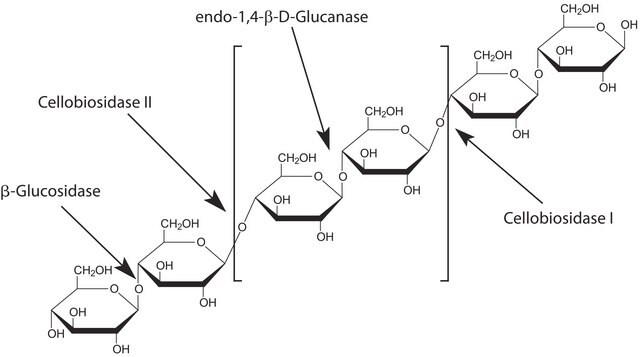
β-N-Acetylglucosaminidase from Streptococcus pneumoniae
recombinant, expressed in E. coli, buffered aqueous solution
Synonym(s):
β-N-Acetyl-D-hexosaminide N-acetylhexosaminohydrolase, β-N-Acetylhexosaminidase
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
buffered aqueous solution
specific activity
≥80 units/mg protein
packaging
vial of ≥1.0 unit
foreign activity
β-galactosidase, α-mannosidase, α-fucosidase, neuraminidase, and proteases, none detected (Enzyme is expressed in glycosidase-free host.)
shipped in
wet ice
storage temp.
2-8°C
Gene Information
Streptococcus pneumoniae R6 ... lytB(934406)
Application
Biochem/physiol Actions
Unit Definition
Physical form
inhibitor
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Learn about O-linked glycan strategies, such as the actions of O-glycosidase, how to remove di and trisialylation sialic acid residues, β-linked galactose, and N-acetylglucosamine, as well as other O-glycan modifications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service