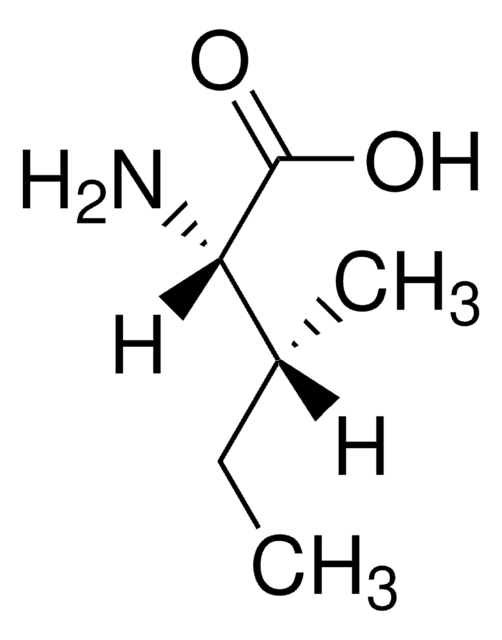
I2752
L-Isoleucine
≥98% (HPLC)
Synonym(s):
(2S,3S)-2-Amino-3-methylpentanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(7)
About This Item
Linear Formula:
C2H5CH(CH3)CH(NH2)CO2H
CAS Number:
Molecular Weight:
131.17
Beilstein:
1721792
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product name
L-Isoleucine, reagent grade, ≥98% (HPLC)
grade
reagent grade
Quality Level
Assay
≥98% (HPLC)
form
powder
color
white
mp
288 °C (dec.) (lit.)
solubility
1 M NH4OH: 50 mg/mL
application(s)
detection
SMILES string
CC[C@H](C)[C@H](N)C(O)=O
InChI
1S/C6H13NO2/c1-3-4(2)5(7)6(8)9/h4-5H,3,7H2,1-2H3,(H,8,9)/t4-,5-/m0/s1
InChI key
AGPKZVBTJJNPAG-WHFBIAKZSA-N
Looking for similar products? Visit Product Comparison Guide
Application
L-Isoleucine has been used in capillary feeder (CAFE) assay in a study to determine the consumption of amino acids by Drosophila melanogaster. It has also been used as one of the components of an amino acid mixture, which is used as a supplement for synthetic complete (SC) media.
Biochem/physiol Actions
L-Isoleucine is an isomer of L-leucine and is an essential amino acid. It is synthesized from threonine and is a branched-chain hydrophobic amino acid.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Amino Acid Biosynthesis Is Regulated by Feedback Inhibition
Biochemistry (5th Edition) null
Inhibitory effect of aroma on the bitterness of branched-chain amino acid solutions.
Mukai J
Chemical & Pharmaceutical Bulletin, 55(11) (2007)
Michel Fleck, Aram M. Petrosyan
Salts of Amino Acids: Crystallization, Structure and Properties (2014)
Large-scale Phenotypic Profiling of Gene Deletion Mutants in Candida glabrata
Fabian Istel
Bio-protocol (2015)
Conversion of ammonia or urea into essential amino acids,L-leucine,L-valine, andL-isoleucine using artificial cells containing an immobilized multienzyme system and dextran-NAD+
Applied Biochemistry and Biotechnology, 26 (1990)
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service