14795
N,N,N′,N′-Tetramethyl-1,8-naphthalenediamine
purum, ≥99.0% (NT)
Synonym(s):
1,8-Bis(dimethylamino)naphthalene, DMAN, Proton-sponge®
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H18N2
CAS Number:
Molecular Weight:
214.31
Beilstein:
396782
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥99.0% (NT)
form
solid
mp
45-49 °C
functional group
amine
SMILES string
CN(C)c1cccc2cccc(N(C)C)c12
InChI
1S/C14H18N2/c1-15(2)12-9-5-7-11-8-6-10-13(14(11)12)16(3)4/h5-10H,1-4H3
InChI key
GJFNRSDCSTVPCJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N,N,N′,N′-Tetramethyl-1,8-naphthalenediamine was used in protonation of multiple-charged oligonucleotide anions. It was used as reagent during reaction of benzaldehyde with acetic anhydride catalyzed by bismuth nitrate. It was used as extractant during separation of organic acids from fermentation broths by liquid-liquid extraction.
Other Notes
Strong amine base ("proton sponge") with unusual properties
Legal Information
Proton-sponge is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R.L. Benoit et al.
Canadian Journal of Chemistry, 65, 996-996 (1987)
Yu Xia et al.
Analytical chemistry, 77(11), 3683-3689 (2005-06-01)
A single sonic spray source has been used to generate both positive and negative ions for subsequent ion/ion reaction experiments. Ion/ion reactions took place after ions of each polarity were sequentially injected into a linear ion trap, where axial trapping
R.W. Alder et al.
Journal of the Chemical Society. Chemical Communications, 723-723 (1968)
Extractant screening for liquid-liquid extraction in environmentally benign production routes.
Krzyzaniak A, et al.
Chemical Engineering Transactions, 24, 709-714 (2011)
Bismuth compounds in organic synthesis. Bismuth nitrate catalyzed chemoselective synthesis of acylals from aromatic aldehydes.
Aggen DH, et al.
Tetrahedron, 60(16), 3675-3679 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service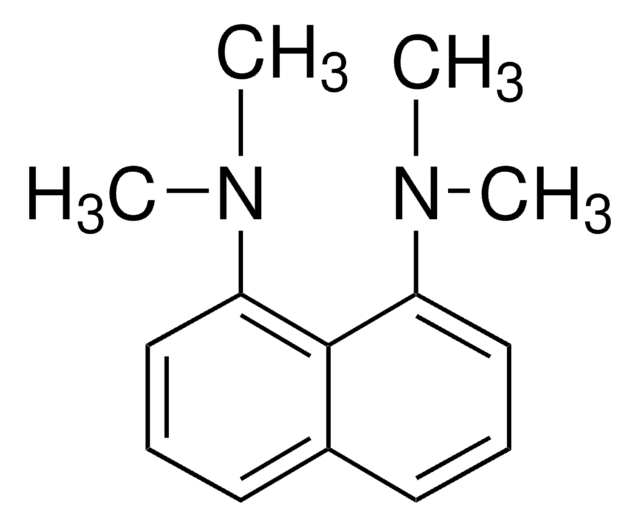
![2,8,9-Triisopropyl-2,5,8,9-tetraaza-1-phosphabicyclo[3,3,3]undecane](/deepweb/assets/sigmaaldrich/product/structures/387/021/edaffe12-6e4b-4305-9030-749551ac828a/640/edaffe12-6e4b-4305-9030-749551ac828a.png)
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)
![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)
![Bis[2-(N,N-dimethylamino)ethyl] ether 97%](/deepweb/assets/sigmaaldrich/product/structures/372/323/505a46ae-b067-4177-8e5f-19a3f4ef9c44/640/505a46ae-b067-4177-8e5f-19a3f4ef9c44.png)
![1,5,7-Triazabicyclo[4.4.0]dec-5-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/171/446/333d560c-cff6-4958-b489-5acfb3057cce/640/333d560c-cff6-4958-b489-5acfb3057cce.png)


![3,3,6,9,9-Pentamethyl-2,10-diazabicyclo[4.4.0]dec-1-ene ≥96.0% (GC)](/deepweb/assets/sigmaaldrich/product/structures/203/819/340f3f5a-eaa1-4393-8425-631460e3154d/640/340f3f5a-eaa1-4393-8425-631460e3154d.png)