E-075
Estrone solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
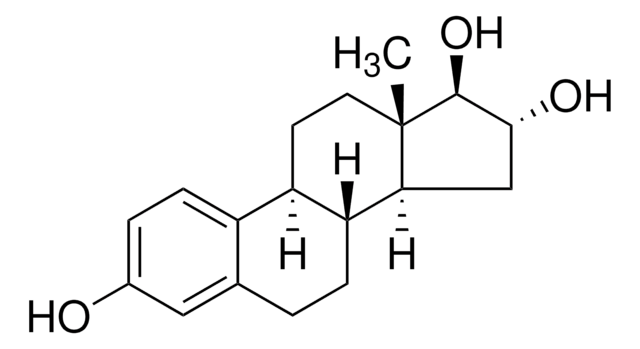
CC12CCC3C(CCc4cc(O)ccc34)C1CCC2=O
InChI
1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChI key
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Gene Information
human ... ESR1(2099)
General description
Application
- Fluconazole solution for yeast infection treatment: Fluconazole is extensively utilized for its efficacy against yeast infections, specifically vulvovaginal candidiasis. It operates by inhibiting the synthesis of ergosterol, crucial for fungal cell membrane integrity, thereby exerting its antifungal effects (Picheta et al., 2024).
- Antifungal medication in systemic candidiasis: Fluconazole solution is critical in treating systemic candidiasis, offering a potent antifungal mechanism that disrupts the fungal cell membrane′s ergosterol content, essential for maintaining fungal cell viability (Dettlaff et al., 2024).
- Fluconazole in vitro antifungal activity assay development: Fluconazole is utilized in the development of in vitro antifungal activity assays, which are crucial for screening potential antifungal agents and assessing their efficacy against various fungal pathogens (Teba et al., 2024).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Estriol 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) 17-sulfate dipotassium salt; Estriol 3-sulfate sodium salt; β-Estradiol 3,17-disulfate dipotassium salt, ≥95%; β-Estradiol 17-(β-D-glucuronide) sodium salt; β-Estradiol 3-(β-D-glucuronide) sodium salt; Estrone 3-(β-D-glucuronide) sodium salt; β-Estradiol 3-sulfate sodium salt, ≥93%; Estriol, ≥97%; Estrone 3-sulfate sodium salt, contains ~35% Tris as stabilizer; β-Estradiol, ≥98%; α-Estradiol, powder, ≥98% (TLC); Estrone, ≥99%
Related Content
The Titan C18 column provided efficient and rapid resolution of thirteen related estrogenic compounds. Ultra Ultra high purity solvents provided robust operation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service