T71803
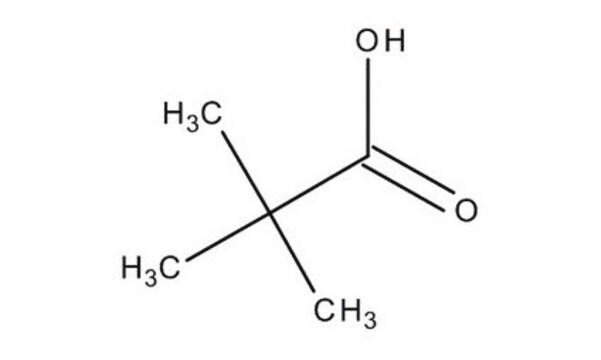
Pivalic acid
99%
Synonym(s):
2,2-Dimethylpropionic acid, Trimethylacetic acid
About This Item
Recommended Products
vapor density
3.6 (vs air)
Quality Level
vapor pressure
9.75 mmHg ( 60 °C)
Assay
99%
reaction suitability
reaction type: C-H Activation
bp
163-164 °C (lit.)
mp
32-35 °C (lit.)
density
0.889 g/mL at 25 °C (lit.)
functional group
carboxylic acid
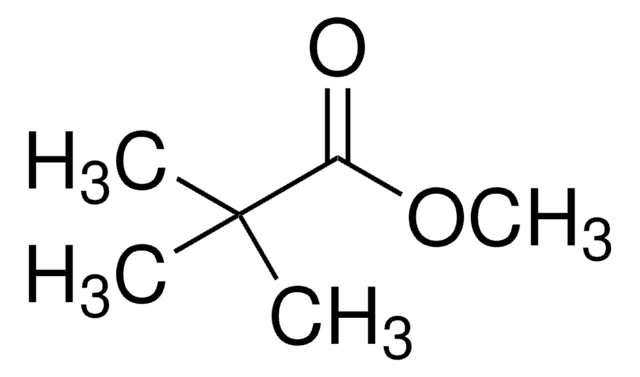
SMILES string
OC(C(C)(C)C)=O
InChI
1S/C5H10O2/c1-5(2,3)4(6)7/h1-3H3,(H,6,7)
InChI key
IUGYQRQAERSCNH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- As a co-catalyst with palladium for the arylation of unactivated arenes and N-heterocycles.
- As an additive to facilitate the carbonylative suzuki reactions to synthesize biaryl ketones from aryl iodides and arylboronic acids by using palladium nanoparticles as catalyst.
- In the cyclization reaction of benzamides with alkynes to synthesize isoquinolones in the presence of 8-aminoquinoline ligand and cobalt catalyst.
Caution
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| T71803-5ML | 4061837372360 |
| T71803-100G | |
| T71803-500G | |
| T71803-500ML | 4061837372353 |
| T71803-100ML | 4061837372346 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service