D99234
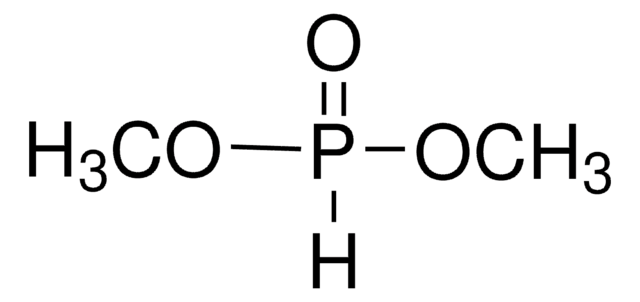
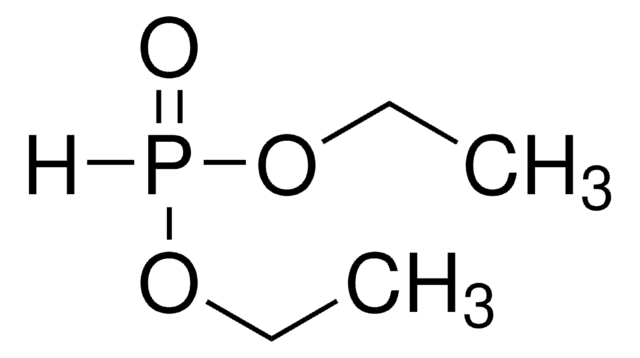
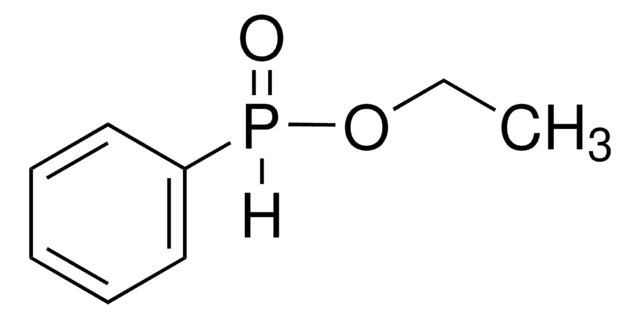
Diethyl phosphite
98%
Synonym(s):
Diethoxyphosphine oxide, Diethyl acid phosphite, Diethyl hydrogen phosphite, Diethyl phosphonate, Hydrogen diethyl phosphite
About This Item
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
50-51 °C/2 mmHg (lit.)
density
1.072 g/mL at 25 °C (lit.)
SMILES string
[H]P(=O)(OCC)OCC
InChI
1S/C4H11O3P/c1-3-6-8(5)7-4-2/h8H,3-4H2,1-2H3
InChI key
MJUJXFBTEFXVKU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- It can undergo condensation with aldehydes or ketones and an amine to form α-aminophosphonates under solvent-free and catalyst-free conditions.
- Reduction of gem-dibromo derivatives using diethyl phosphite in the presence of triethylamine gives monobromocyclopropanes.
- Along with 4-dimethylaminopyridine, it can be used as a ligand for nickel-catalyzed cross-coupling of aryl and heteroaryl bromides, chlorides or sulfonates with arylzinc reagents.
- It can be used for the catalytic asymmetric hydrophosphonylation of enones in the presence of a dinuclear zinc complex to form γ-oxo-phosphonates.
- DEP can undergo Michael addition to α,β-unsaturated malonates to form β-phosphonomalonates in the presence of recyclable nano γ-ferric oxide-pyridine based catalyst.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Sens. 1B
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
179.6 °F - closed cup
Flash Point(C)
82 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service