D107204
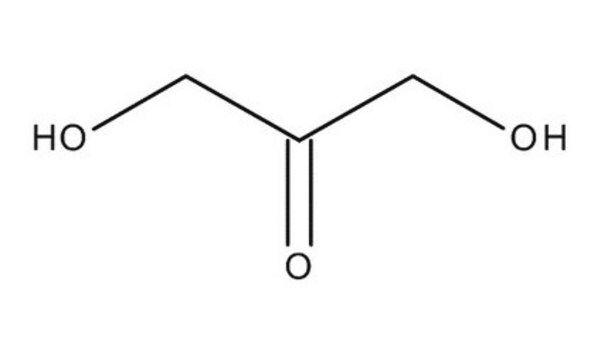
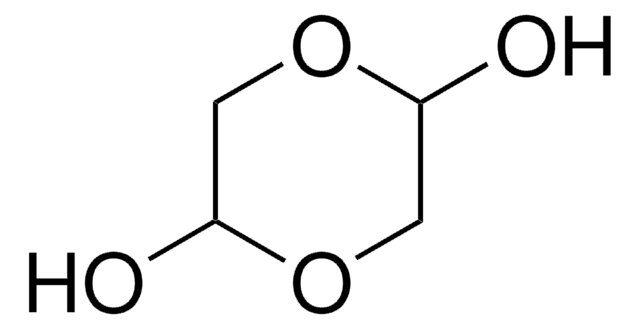
1,3-Dihydroxyacetone dimer
97%
Synonym(s):
2,5-Dihydroxydioxane-2,5-dimethanol
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C6H12O6
CAS Number:
Molecular Weight:
180.16
Beilstein:
112910
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
75-80 °C (lit.)
storage temp.
2-8°C
SMILES string
OCC1(O)COC(O)(CO)CO1
InChI
1S/C6H12O6/c7-1-5(9)3-12-6(10,2-8)4-11-5/h7-10H,1-4H2
InChI key
KEQUNHIAUQQPAC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1,3-Dihydroxyacetone dimer can be used as a precursor to synthesize:
- Nitric acid esters such as 1,3-dinitratoacetone and 2,5-bis(nitratomethyl-2,5-nitrato)-1,4-dioxane.
- Lactic acid in the presence of aluminum salts as catalysts.
- Phosphorus doped carbon quantum dots which can be used as fluorescence labels for fingerprints imaging.
- 1-Methyl-5-hydroxymethylimidazole scaffolds.
Substrate for galactose oxidase.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Reagent-depending Nitration of 1, 3-Dihydroxyacetone Dimer.
Hermann TS.
Zeitschrift fur Anorganische und Allgemeine Chemie, 643(2), 149-151 (2017)
Ana Vrsalović Presečki et al.
Bioprocess and biosystems engineering, 41(6), 793-802 (2018-02-22)
The stereoselective three-enzyme cascade for the one-pot synthesis of (1S,2S)-1-phenylpropane-1,2-diol ((1S,2S)-1-PPD) from inexpensive starting substrates, benzaldehyde and acetaldehyde, was explored. By coupling stereoselective carboligation catalyzed by benzoylformate decarboxylase (BFD), L-selective reduction of a carbonyl group with alcohol dehydrogenase from Lactobacillus
P-doped carbon nano-powders for fingerprint imaging.
Algarra M, et al.
Talanta, 194(5), 150-157 (2019)
New substrate for galactose oxidase.
G T Zancan et al.
Biochimica et biophysica acta, 198(1), 146-147 (1970-01-14)
Dihydroxyacetone conversion into lactic acid in an aqueous medium in the presence of metal salts: influence of the ionic thermodynamic equilibrium on the reaction performance.
Jolimaitre E, et al.
Catalysis Science & Technology, 8(5), 1349-1356 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service